The Small Incidental Renal Mass: Treatment Options
I have summarized and edited presentations from an April 2012 Kidney Cancer Association Patient Conference so patients can read and study them.
The original presentations can be found on YouTube offered by the KCA.
Poor resolution slides have been reconstructed
Treatment Options for the Small Incidental Renal Mass
After a brief welcome and expression of appreciation for the KCA, Dr. Karam begins:
“Currently, a small renal mass is defined as smaller than 3 or 4 cm, localized to the kidney, not invasive to other organs. This is a consensus and these definitions can change. Of all Stage I kidney tumors, 43 % are less than 3cm in size. (Some Stage I kidney cancers can be larger than 3cm; other treatments may be more suitable.)
I will discuss three options with patients who present with these types of lesions. The first is active surveillance, the least invasive, the next is energy ablation, and the third is surgical removal. Each will be discussed in the sequence.
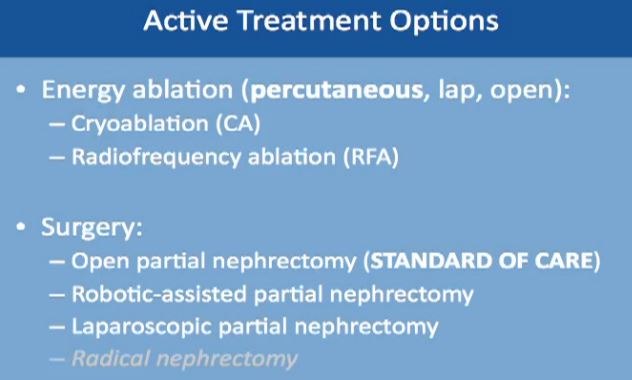
First “Active Treatment Options” is energy ablation. This can be done through the skin, percutaneously, laporascopically, or be done “open”. Energy ablation includes two categories, Cryoablation (CA)–freezing the tumor, done laporascopically or through the skin. Alternatively is Radiofrequency Ablation (RFA), typically done percutaneously, or through the skin.
The second major category of active treatment is surgery, the standard of care being an open partial nephrectomy. That removes just the tumor, leaving most of the kidney. It is now more often being done as a robotically assisted partial nephrectomy. Another option is to do it laporascopically, with minimal incisions. Radical nephrectomy, complete removal of the kidney along with the tumor is the lowest on the list. It is the least favorable option, as we try to save the kidney, wherever possible.
AUA (American Urological Association) Guidelines have published some guidelines, based on multiple studies and experts. The standard of care for treatment of small kidney masses is partial nephrectomy. A radical nephrectomy is used only if the mass cannot be removed by partial nephrectomy. A recommendation could be ablation or active surveillance. This will be tailored to individual needs of the patient and the kidney, as to the location of the mass and its characteristics. No one recommendation fits all patients, and each must be discussed and individually tailored.
Next is active surveillance. We don’t use “watchful waiting”, but “Active Surveillance”. We do not to tell someone to go home with your small mass as we don’t need to do anything about it. On the contrary, we have to actively bring the patient to the clinic for CT scans.
What is active surveillance and what does it entail? That means doing no interventions, no surgery, and no procedures that are invasive–active surveillance. Initially we would do imaging at 3-6 months intervals in the first year, subsequently imaging at 6-12 months. There are no rigid guidelines at this point. I always tell my patients to be aware that we have only had small studies with active surveillance, and with short follow-up of 2-3 years. There is a very small potential for spread or metastases while we are doing active surveillance, so every patient has to be informed about all of this.
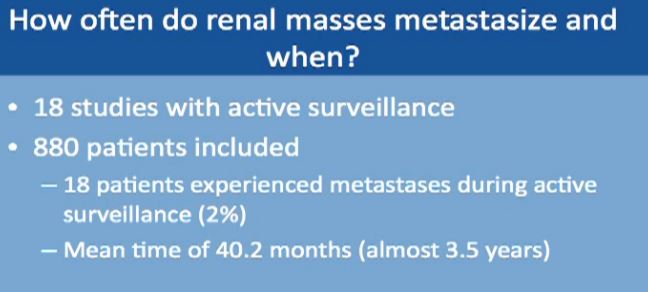
How often do small masses spread or metastasize? When does it seem to happen? This is a meta analysis of data from 18 studies with almost 900 patients. In only 18 patients of 880 did metastases occur while on active surveillance, a rate of about 2%. This spread occurred almost 3 ½ years after initiation of surveillance, so it does not occur in the first 3-6 months, more on average about 3 ½ years.
“How fast does a mass actually grow?” We know that 23% do not grow at all within 2 years or so. The rest do grow at the growth rate shown.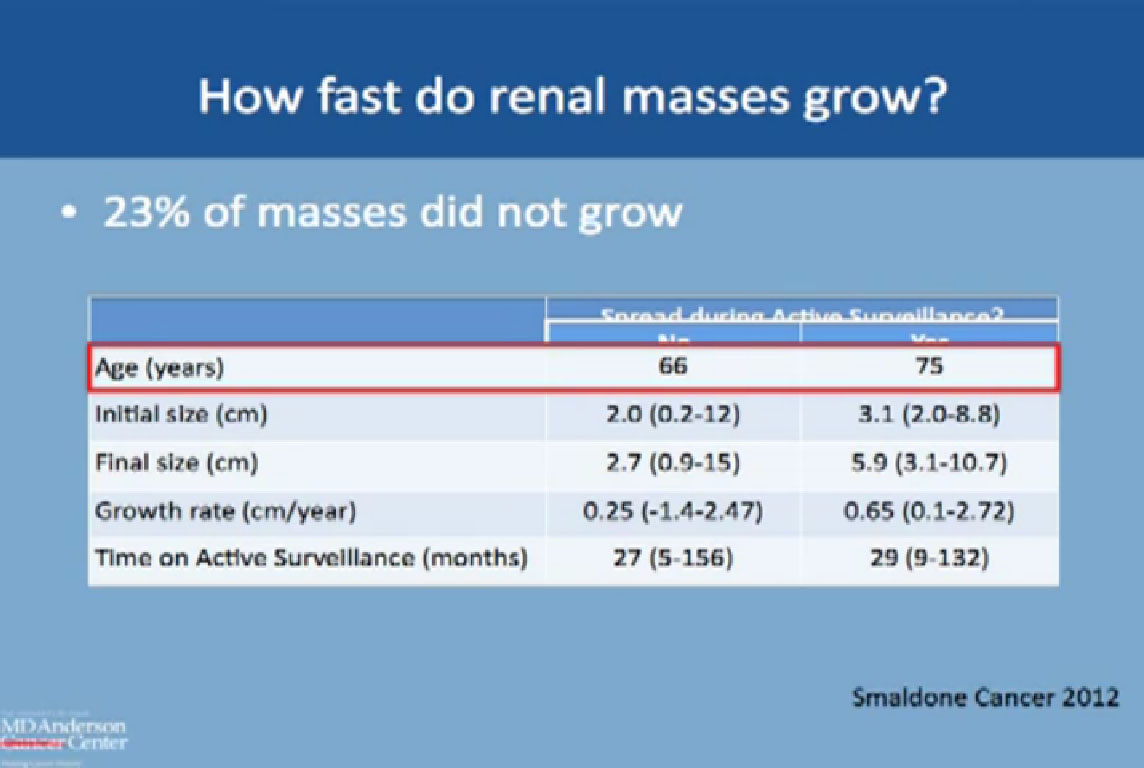 Comparing the growth rate of those who had spread or metastases with those who had none, only 18 patients had spread. The group without spread was typically younger (66 versus 75), with a smaller mass at the initial presentation, 2cm versus 3 cm. The final size in centimeters of those who had spread, those 18 patients, was about 5.9cm. The tumor was quite large at the time when the metastases occurred, and here we started at a size of 3.1 cm.
Comparing the growth rate of those who had spread or metastases with those who had none, only 18 patients had spread. The group without spread was typically younger (66 versus 75), with a smaller mass at the initial presentation, 2cm versus 3 cm. The final size in centimeters of those who had spread, those 18 patients, was about 5.9cm. The tumor was quite large at the time when the metastases occurred, and here we started at a size of 3.1 cm.
The growth rate was about 2mm per year for the patients who did not have spread, and about 6mm per year for patients who did have spread. The time on active surveillance was about 2 ½ years.
Of these patients on active surveillance, what happened to them, did they all continue after active surveillance? Over half did remain on active surveillance, over a period of 2-3 years. Fewer than half had delayed intervention. They might have gone to ablation or surgery, and this is over a period of 2-3 years. Others had “delayed intervention, with patients having ablation or surgery on average about two years after starting active surveillance.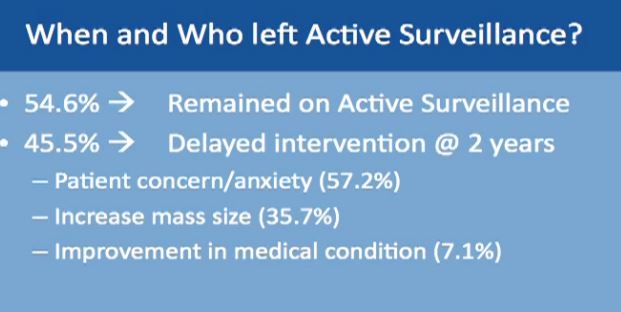
The common reason for moving from surveillance to intervention was typically patient concern about the growing mass—patient anxiety. The next reason was enlargement of the mass and the third one was that a patient had some bad medical problem initially not related to the mass. When that problem was solved, and became a good surgical candidate and they are switched to surgery.
Energy Ablation: Radiofrequency
Next is Energy Ablation, a more invasive category which includes radiofrequency and cryoablation.
How does radio frequency ablation work? I usually tell my patients that is basically frying the tumors. The waves from radiofrequency cause friction and movement in the water molecules. This produces heat and destroys the tumor. Cell death occurs in about five minutes after exposure to the RF, with the temperature over 50 degrees Celsius )122 Fahrenheit. This can be monitored during the procedure. We have to achieve temperatures up to 100 (212 F)degrees in order to effectively destroy the tumor with this procedure.
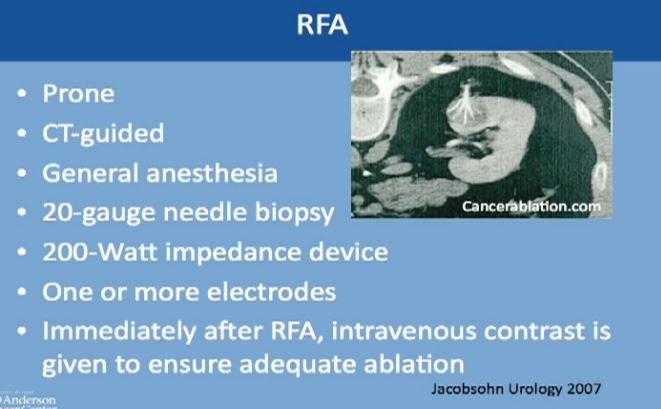
This is a CT scan of the vertebral column and the patient is laying flat on this tummy. This is the kidney, this is the mass, and is CT guided. The interventional radiologist usually puts this thick needle with these tines, typically done under general anesthesia. Though we can do it very infrequently under conscious sedation, but general anesthesia is preferred. A biopsy is typically done at that time and one or more electrodes are inserted. Immediately after the procedure iss done, we do a CT to see that there isn’t any bleeding and to make sure this was treated adequately.
Energy Ablation: Cryolablation
(This is a video which can be seen on the Youtube video.)
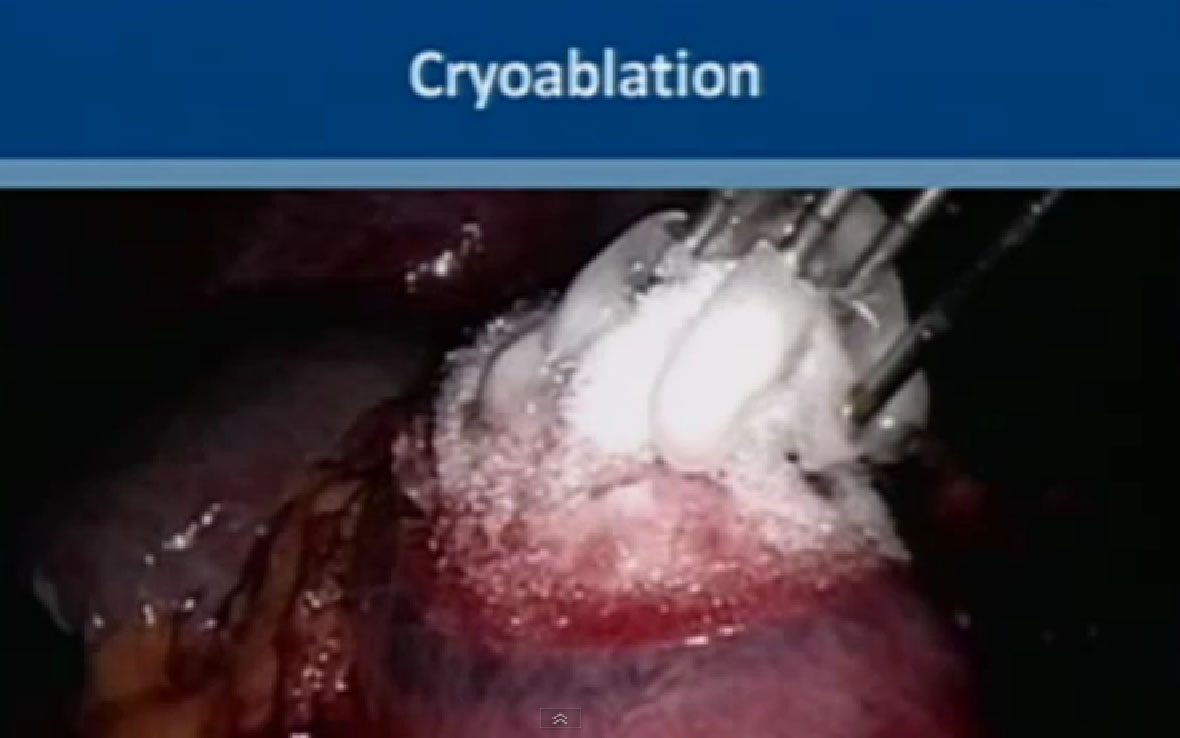
The next category of energy ablation is cryoablation or freezing the tumor. This is a picture during laparascopic surgery. You can see them freezing the tumor. This is the kidney here, the tumor here. The tumor here has been marked; the electrodes that go into the tumor, freeze the tumor and form an ice ball.
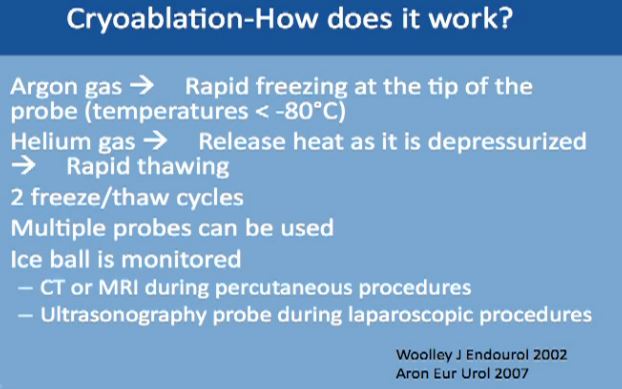
We use two gases for this procedure. The first is argon, which causes rapid freezing at the tip of the probes, with the temperature dropping to less than 80 degrees Celsius. The second gas is helium, which releases heat as it depressurizes, and it causes very fast thawing of the tumor. This typically repeated twice, two cycles of freezing and thawing. Multiple probes can be used to achieve tumor ablation. With this technique the ice ball can be monitored and you can see the progress with time. This can be monitored by CT or MRI, done percutaneously under guidance, or with an ultrasound when you are doing it laporascopically. or with an ultrasound when you are doing it laporascopically.
 Cryoablation—How Does It work?
Cryoablation—How Does It work?
The Cyroablation acts directly on the tumor cells. It dehydrates the cancer cells, removes all the water from them, which causes damage to the enzymes which are needed for survival by the cells, the organelles and the cell membranes. It also causes the formation of ice inside the cell and kills the cell. But it can also act indirectly, as it targets the cells directly, cutting off nutrition needed for growth. In order to achieve this, very low temperature have to be reached, and to achieve it homogeneously for all of the tumor, the ice ball must extend ½ to 1 cm beyond the margin of tumor to make sure it is completely frozen.
Cryoablation
As to follow up, how do we know it is still working? There will be routine visits to the clinic with medical history, physical exams, chest imaging which is either with conventional radiography, CT and routine blood work.
Follow Up
To follow up on the tumor itself, we typically do a CT or MRI with IV contrast to visualize the tumor. The first follow up is at 6 weeks after the ablation to make sure the ablation was complete and successful and then at 6, 12, 18 and 24 months after ablation.
It does not mean that follow up will end at 24 months. The patient continues with scans for life, routinely, at least once a year after deletion or the treated tumor is stable. So imaging has to continue for years, as we don’t have long term studies with these technologies.
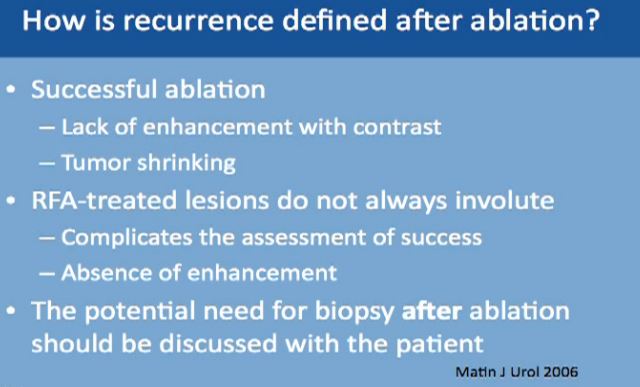
We tend to do more biopsies if the mass is not shrinking well enough, or if it is taking contrast, or it doesn’t look right one year. If the mass is not looking right, we go ahead and do the biopsy to make sure there isn’t any recurrence.
Recurrence defined after ablation can be tricky because the mass is still there. A successful ablation is defined as lack of enhancement of the tumor with the use of contrast. That is why is it very important for the patient to receive and tolerate contrast IV contrast for follow up after ablation. With cryoablation, typically you see tumors shrinking. With Radio Frequency, it doesn’t always happen. That is what makes it trickier, so we have to do biopsies for patients after treatment. And again, the tumor does not always shrink; more typically it does with the cryoablations. Again, radio frequency ablation lesions don’t always shrink, so absence of enhancement is what we look for which is not always ideal.
Now we always discuss with our patients the potential need for biopsy after ablation so this may be expected, though is not routine. Thus, the patient has to be aware of this. We don’t do it routinely, but it is a possibility.
 What can predict who is going to have a successful ablation? Tumor size less than 3 or 4 cms is a very good indicator that we will be able to successfully ablate the tumor. If the tumor is exophytic, that the tumor is not deep into the kidney, this is a good indicator. If the tumor is peripheral or not central, that means that the tumor is away from the artery and the vein of the kidney. This is also a very good indicator that we can successfully treat it with ablation.
What can predict who is going to have a successful ablation? Tumor size less than 3 or 4 cms is a very good indicator that we will be able to successfully ablate the tumor. If the tumor is exophytic, that the tumor is not deep into the kidney, this is a good indicator. If the tumor is peripheral or not central, that means that the tumor is away from the artery and the vein of the kidney. This is also a very good indicator that we can successfully treat it with ablation.
 There are some complications, including pain after the procedure. This is usually an overnight stay; the patient goes home the next day. There could be some numbness or neuromuscular complications. There can be some air around the lung, if the needle had to be inserted close to the lung. This is typically monitored during the procedure.
There are some complications, including pain after the procedure. This is usually an overnight stay; the patient goes home the next day. There could be some numbness or neuromuscular complications. There can be some air around the lung, if the needle had to be inserted close to the lung. This is typically monitored during the procedure.
Seeding from the tumor is exceedingly rare, maybe 1 or 2 cases, so exceedingly rare, nearly unheard of, so this should not be a concern for patients who are willing to go through this procedure. Bleeding can occur, and that‘s why we like to keep patients overnight. Also there could be some strictures or narrowing of the ureter tube that drains the kidney.
So how do we know to recommend RFA or Cryoablation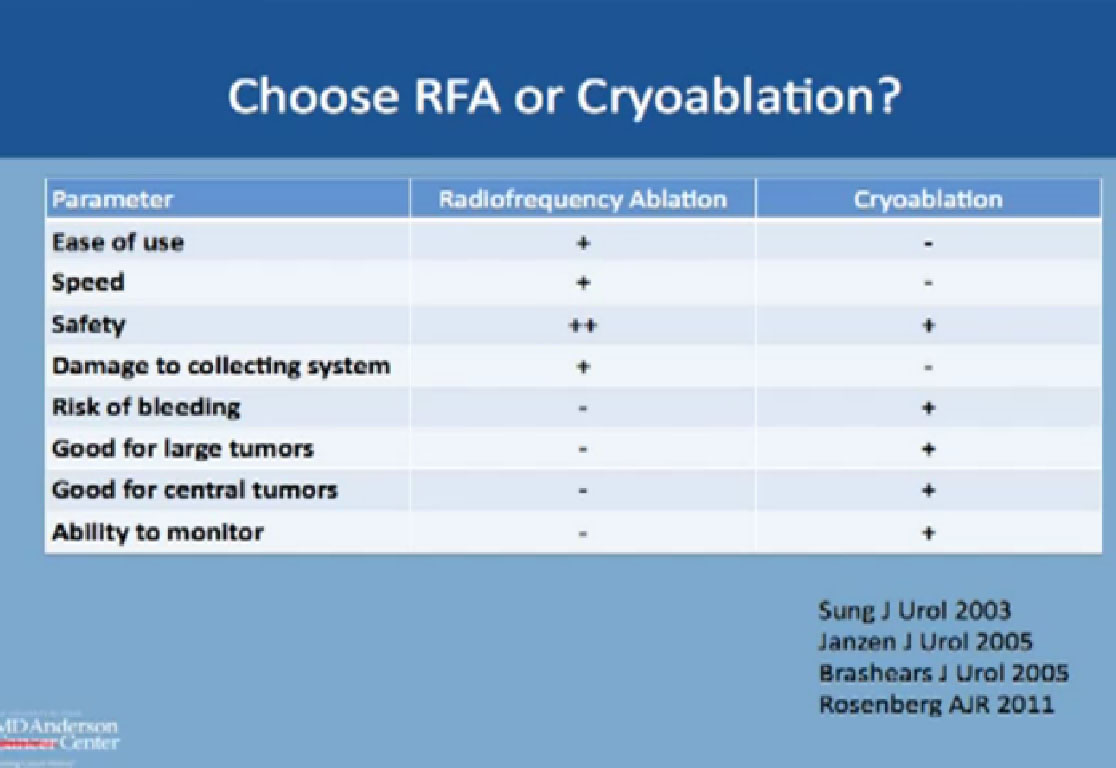 ?
?
We know RFA is considered easier, faster, and safer, but actually has a higher chance of doing damage to the collecting system, the system that drains urine from the kidney. On the other hand, CA has a slightly higher risk of bleeding compared to RF. But it is better for larger tumors, and is good for deep tumors or central tumors. You can monitor live CA with ultrasound or CT to see the ice ball actually grow and treat the whole tumor.
Partial Nephrectomy
The more invasive category is the partial nephrectomy, the actual removal of the tumor, leaving the rest of the kidney in place. The standard had been the open procedure, but nowadays, people tend to do more robotic assisted surgery. You have to have a good surgical candidate for this, both as to the patient’s anatomy and the actual mass. Less commonly used is the pure laparoscopic, just because it is technically challenging and because most hospitals have a robot available.
(VIDEO available on Youtube, about 17 minutes into clip.)
We will show a two minute clip of robotic nephrectomy surgery, courtesy of my colleague. The kidney is here, artery to the kidney here and the vein to the kidney is here. First we need to stop the blood flow to the kidney to do this procedure safely and accurately. A clamp is temporarily placed on the artery and another clamp on the vein, which will completely shut off the blood supply. Here the tumor is literally being carved out of the kidney. Here is the tumor, this is normal kidney, this is tumor, and this is the tumor over here, normal kidney here, so this is what is considered a partial nephrectomy. We check that the mass is excised and then suture the kidney so we can get normal control and prevent bleeding. All you see here is normal kidney. (This is on slightly fast forward–we don’t typically work this fast!)
So now they are bringing back the edges of the kidney together. We use some substance to prevent bleeding which will dissolve on their own: bringing the edges of the kidney back together. And you can see very early on that very little of the normal kidney is removed, just to give a margin to assure that we remove the whole tumor. We remove all the clamps to bring back normal blood supply to the kidney, so this is how the kidney looks. It is pink because it has the normal blood supply. There is no bleeding seen.
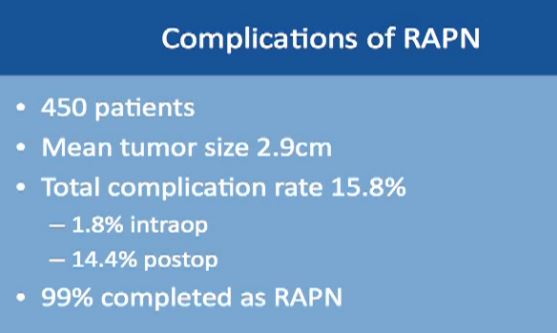
Complications might occur in some procedures. This is a large study of almost 500 robotic-assisted partial nephrectomies. Tumor sizes were about 3 cm, so small kidney masses. The total complication rate is about 16%. Complication rates include everything, even slight fevers, so higher than you would expect. Less than 2% of the complications happen during the surgery; most of the complications happen afterwards. About 99% of the surgeries were completed with a robot. Main complications specific to a robotic–assisted partial nephrectomy are bleeding, urinary leakage (1.7%), radical nephrectomy (1.6%), which means removing the whole kidney.
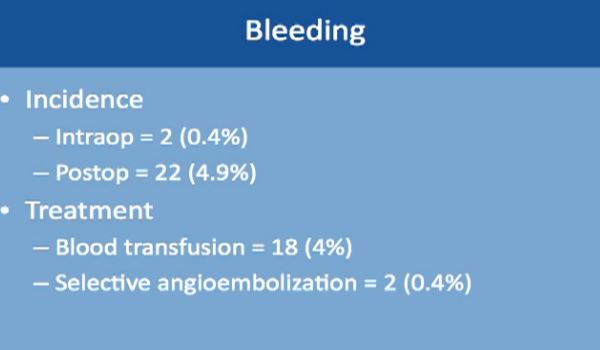
Bleeding occurred in two patients only during the operation, and about 5% of patients while they are still in the hospital. The treatment is relatively simple. Blood transfusion took care of 4% of these patients. About 2% of these patients needed selective embolization, a procedure that is usually done by interventional radiology and done under sedation. This saves the kidney and controls the bleeding as well.
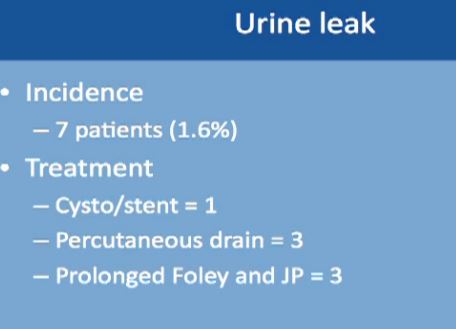
Urinary leakage is found in less than 2%, with treatment typically to put in a stent or a drain or leaving the catheter in longer. None of these patients needed repeat operations or removal of the kidney.
Another complication is conversion to a radical nephrectomy, which occurred in less than 1.6% of patients. This may be due to finding multiple tumors not recognized earlier. In one patient, the tumor could not be found; this happens if the tumor is small and very deep into the kidney. In five patients, a negative margin could not be achieved. That means some cancer was left behind after the tumor was carved out. Then the physician decided to remove the whole kidney to make sure all the cancer was gone. Also, one or more tumors cannot be removed successfully with the kidney left in place. Such complications occur less than 10% under any procedure.
In this table (which could not be captured), but the tumor size was about 3 cm on average. Surgery time was about 3 hours. Blood loss was about 200-300 ccs and hospital stay was about 3 days. This is a different study with complications about 5%. And with positive margins, i.e, a tiny amount of cancer was left, was about 1.6%. The follow up was about four years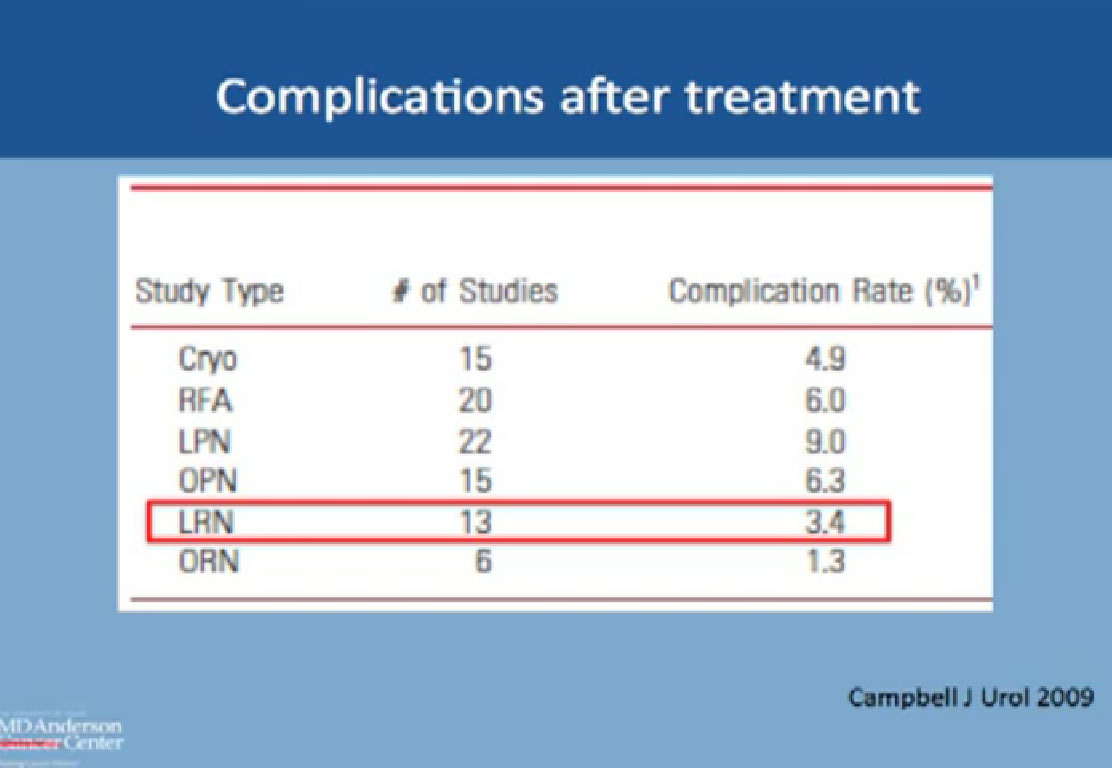 .
.
Complications after Treatment Now to compare the rate of complication with RF and cyroablations and surgery: this is from the AUA guidelines; the complication with Cryoablation is about 5%, with RFA about 6%, about 9% with lap partial, and about 6% with open partial and about 3% with complete removal laporascopically, and about 1% with open. All are less than 10% complications.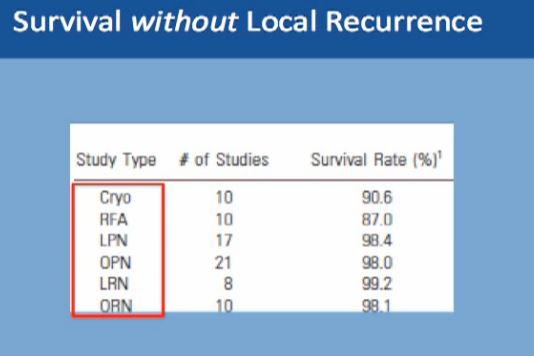
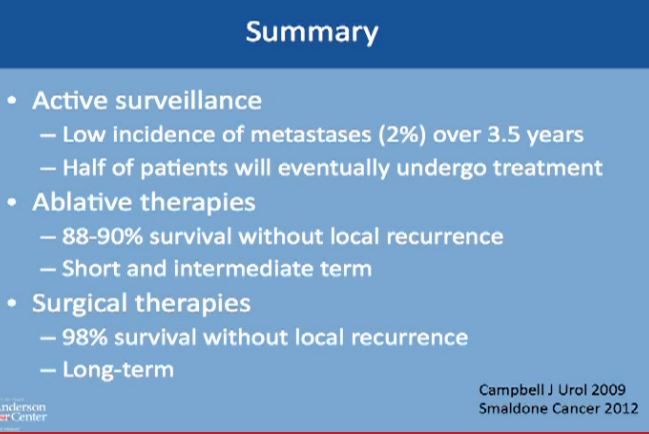
What about survival without a local recurrence?For cryoablation, it is 90% This is not complete survival, this is living without a recurrence. Again, 90% with cryolablation, with RFA about 87%, and with surgery about 98%.
Renal Function
-
Partial nephrectomy and RFA has similar renal functional outcomes
-
No change in GFR
-
No change in CKD stage
Kidney function is pretty much the same after these procedures, whether you do a partial removal of the tumor or an ablation, so that is not a major factor in decisions.
Active surveillance should be offered to appropriate patients. Patients should be informed that metastases could happen, but typically less than 2% of the time, and it typically takes on average 3-4 years on average. before that would happen. Half of all patients will undergo some treatment eventually, and leave active surveillance.
Survival without Local Recurrence
With the second modality, energy ablation with radio frequency or cryoablation, the chances of survival without local recurrence are about 88-90%, and we have intermediate term data, but not long term follow up on those patients.
However, with surgery you have 98% chance of survival without having a local recurrence to that same kidney. With surgery, we do have long term data as to partial nephrectomy.
A partial nephrectomy is the most definitive therapy. Any patient who is willing to undergo surgery and can tolerate surgery should do have partial nephrectomy for small renal masses. Alternatively, we can offer patients surveillance or ablation. We typically reserve that for that for those with poor kidney function—and we don’t to cause more kidney dysfunction, for those with more have multiple tumors, or for the patient at a high surgical risk or with a poor performance status. That latter patient means he might be a poor surgical candidate due to other health issues, with a heart problem or more dangerous tumors, for example. We can offer surveillance or ablation for those patients. If patient does not want surgery, we can offer two other options. This concludes my talk and I welcome questions.”