(The lecture is from a patient conference, and may be seen by clicking through to YouTube. My transcription may make it easier to STUDY the information from Dr. McDermott and to review the slides.)
Dr. David McDermott; Dana Farber Harvard Cancer Center; KCA Conference
“This is a great opportunity to talk about this new research. The last six years brought great advances to the treatment of metastatic kidney cancer with newly approved therapies. No other area of cancer that has had made more progress with new treatments in that time, and there is more good news to come.
In this era of targeted therapies, some have asked if immunotherapy–once the only option for kidney cancer–has any role. In the next twenty minutes, I will show you that it probably still does–for the proper patient.
Dr. Wood talked about limitations with the targeted therapies, the anti-angiogenesis drugs. They are excellent at pruning the tumor, much like I do with my weed whacker. Though they can delay progression, and improve survival, you need to be on treatment to have its effect and all these tumors come back. Once you are off treatment, the effect wears off. They can delay progression, extend life and patients are living longer.
There has been a greater understanding about how the immune system works over the last 20 years when this was introduced.
The Immune System
Adaptive defense system that protects an individual from invading microorganisms
The immune system is specific
Involves multiple large polypeptides that function in:
Antigen presentation
Antigen recognition
Intercellular signaling
Involves multiple cell types Slide 2
These insights can improve outcome for our patients. The immune system is a defense system, which protects the person from invading bacteria and viruses. This system contains multiple moving parts and multiple cell types.
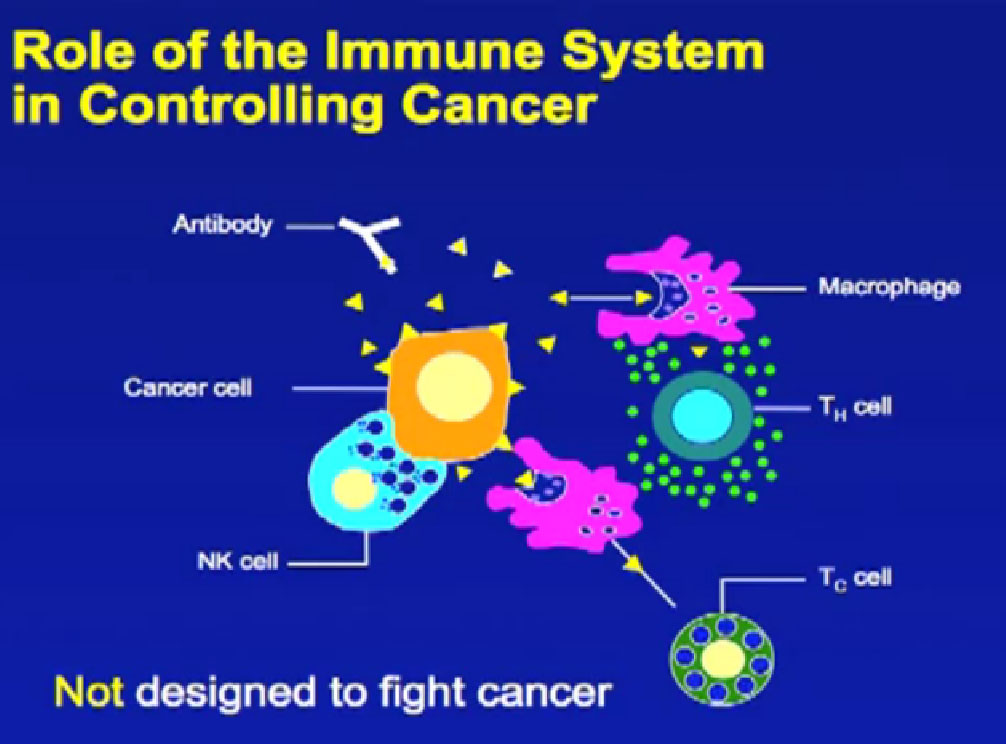 Here is a cartoon example of all the different cells and antibodies, which can protect against bacteria, others that can target a microorganism, or more importantly, a tumor cell. This system was not designed to fight cancer; it was designed to fight infection. That means it is designed to turn on when you have an infection like a virus, and when the virus is controlled, the same “turned on” cells of the immune system are then shut off. These shutoff mechanisms are in part responsible why cancer can evade the immune system, and why it has been so difficult to use the immune system to fight the cancer, as opposed to fight infections.
Here is a cartoon example of all the different cells and antibodies, which can protect against bacteria, others that can target a microorganism, or more importantly, a tumor cell. This system was not designed to fight cancer; it was designed to fight infection. That means it is designed to turn on when you have an infection like a virus, and when the virus is controlled, the same “turned on” cells of the immune system are then shut off. These shutoff mechanisms are in part responsible why cancer can evade the immune system, and why it has been so difficult to use the immune system to fight the cancer, as opposed to fight infections.
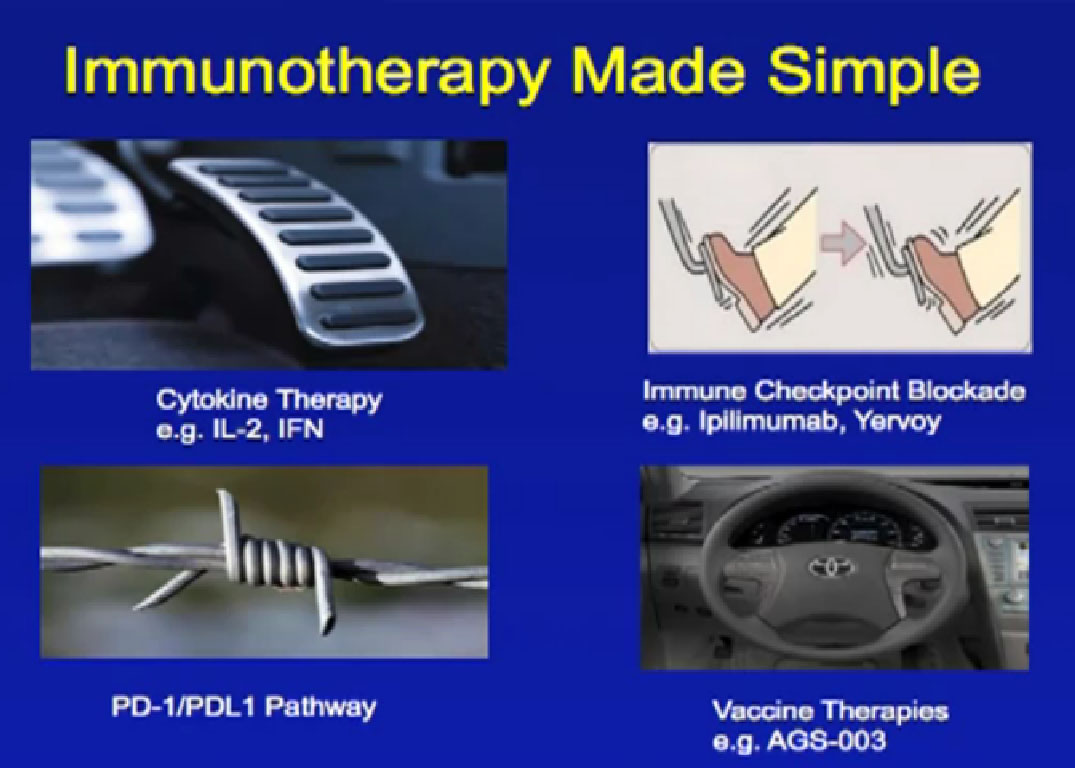 Different approaches are being used with the immune system to fight cancer, some we’ve used in the past and some exciting new approaches currently being tested. The first approach is much like pressing on the gas. This approach to immune therapy has mostly involved cytokines, with interleukin 2 and interferon, and was used for a long time. They rev up the immune system and are growth factors that stimulate a lot of the parts of the immune system, such as T-cells.
Different approaches are being used with the immune system to fight cancer, some we’ve used in the past and some exciting new approaches currently being tested. The first approach is much like pressing on the gas. This approach to immune therapy has mostly involved cytokines, with interleukin 2 and interferon, and was used for a long time. They rev up the immune system and are growth factors that stimulate a lot of the parts of the immune system, such as T-cells.
Dr. Wood said there were no home runs for patients for Stage IV kidney cancer, but that is not exactly true. There are very few home runs, a few patients with stage IV disease who obtain a remission of their disease. Remission means treatment of Stage IV disease in which the cancer goes away, treatment stops, and cancer does not come back. To most patients, that is their goal. Very few patients meet that goal, but for some that remission has lasted decades.
Pressing on the Gas of the Immune System
It is also true that this “pressing on the gas of the immune system” does not work for most patients. It is hard to identify which patients it will work for and is associated with lots of side effects. To be effective, as with interleukin 2, these treatments must be given in a hospital. They involve serious side effects, sometime life-threatening side effects, so the kind of patient who can receive this type of treatment is relatively limited.
At Beth Israel Deaconess in Boston we try to be selective choosing patients for this therapy. We try to identify the patients who may receive the home run, before we put them through the side effects, because so many patients go through the side effects without benefit.
High-Dose Aldesleukin “Select” Trial in Patients with Metastatic RCC
D. McDermott, M Ghebremichael, S Signoretti, K Margolin, J Clark, JSosman, J Dutcher,M French, and M Aktins on behalf of the Cytokine Working Group
We had clues about things that might improve the selection, so created the IL2 SELECT trial, reported at ASCO two years ago. The activity of IL2 in this era of these new treatments is still relatively good, as good as it was 20 years ago. The response rate in this trial with 120 patients was 25%, significantly greater than it was when the drug was introduced 20 years ago. The drug is not any better, or are we any better at giving it. But we are better choosing our patients. Choosing who should get it, and who shouldn’t, and that improves the responses of our patients.
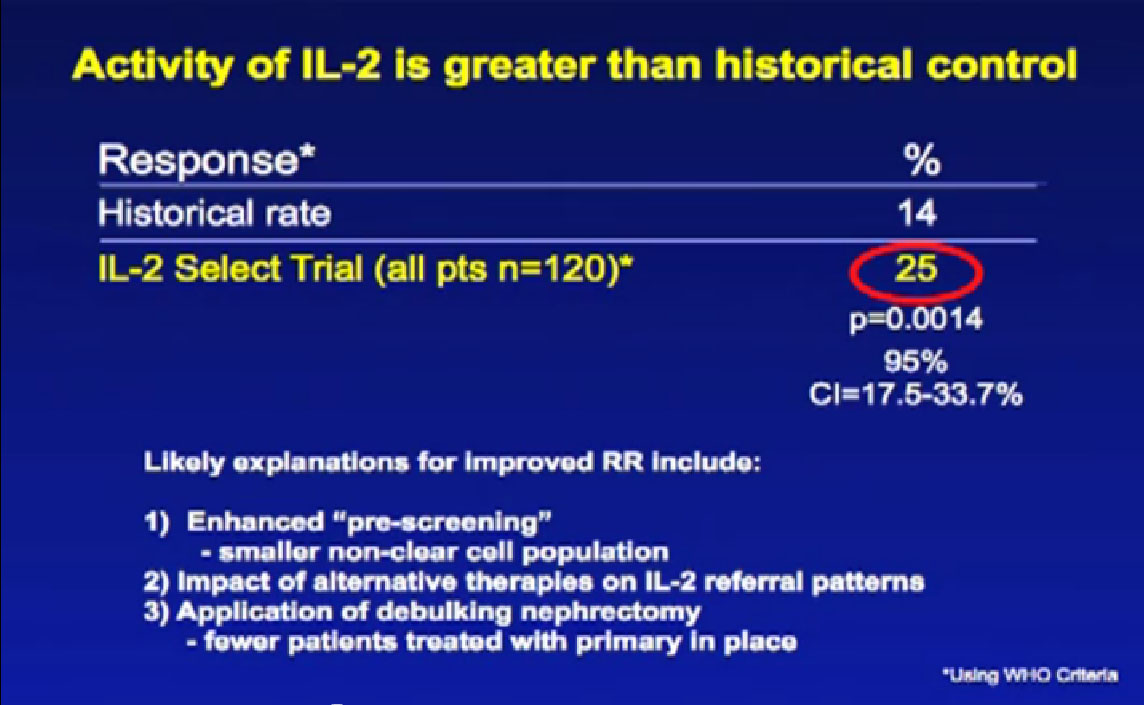 It has been known that patients with non-clear cell are less likely to respond to immunotherapy, so in this trial there were very few patients with non-clear cell cancer—and no responders in that group. It is also known that patients who had their primary tumor removed when they presented at Stage IV disease are more likely to respond to immune therapy. In this trial, 99% of patients had that surgery before they went into this trial. It is almost certainly true, that with new therapies available, that the types of patients being referred to an IL2 centers like ours is probably different. Changing the types of folks we are treat means our numbers are improving somewhat.
It has been known that patients with non-clear cell are less likely to respond to immunotherapy, so in this trial there were very few patients with non-clear cell cancer—and no responders in that group. It is also known that patients who had their primary tumor removed when they presented at Stage IV disease are more likely to respond to immune therapy. In this trial, 99% of patients had that surgery before they went into this trial. It is almost certainly true, that with new therapies available, that the types of patients being referred to an IL2 centers like ours is probably different. Changing the types of folks we are treat means our numbers are improving somewhat.
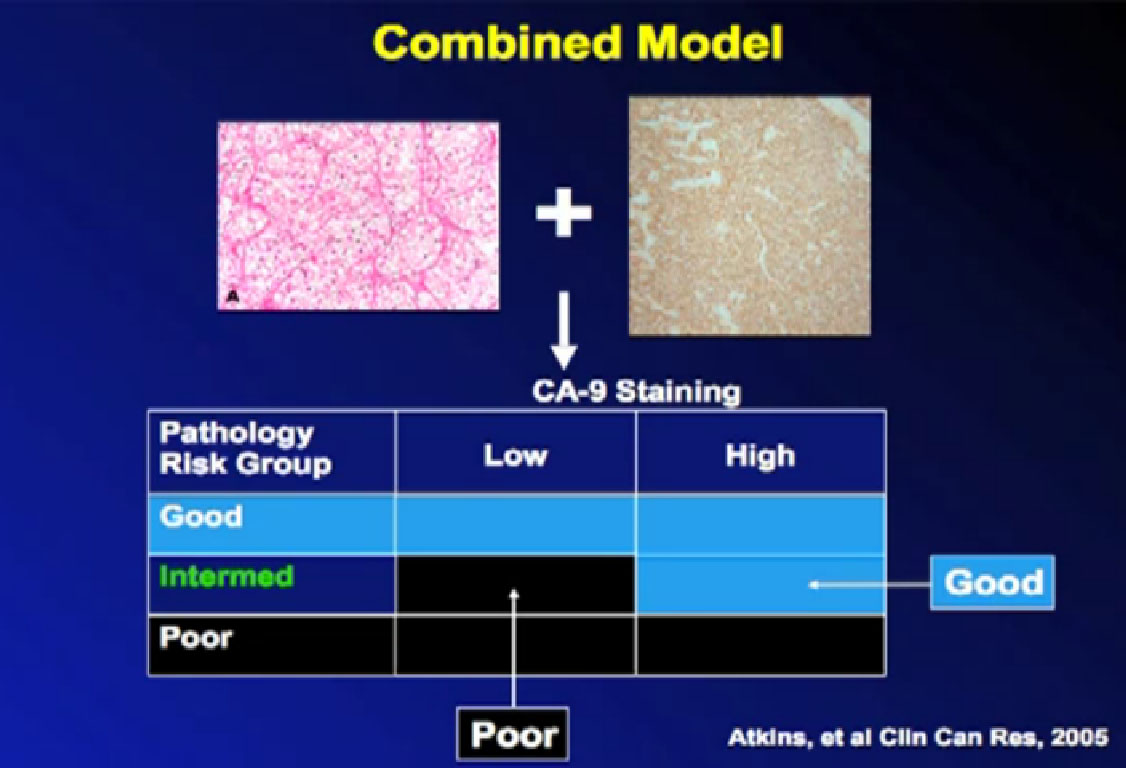 To improve the selection process and based on earlier research, we wanted to show that certain features of the patient’s pathology of the tumor might predict for response. We looked at what the tumor under the microscope and special proteins (CA IX or carbonic anhydrase IX) that the tumor might produce. We thought those things might predict either high chance for response or low chance for responding to treatment.
To improve the selection process and based on earlier research, we wanted to show that certain features of the patient’s pathology of the tumor might predict for response. We looked at what the tumor under the microscope and special proteins (CA IX or carbonic anhydrase IX) that the tumor might produce. We thought those things might predict either high chance for response or low chance for responding to treatment.
Unfortunately for me as a researcher, although fortunately for the patients who went on this trial, the folks who were in the poor risk group–those we thought would not benefit from IL2–did just as well as those not in the poor risk group. It surprised us and showed the importance of testing theories in the proper trials. We clearly need to do more work in this direction to find something to explain why some people benefit and some do not. And it may turn out that response may have more to do with the patient’s immune system than the cancer as how they benefit from treatments that boost the immune response to cancer.
RCC IL-2 SELECT Trial; Conclusions
Tumor features did not predict for response.
Efforts to confirm other predictors are ongoing.
Lessons from this work may guide the development of targeted immunotherapies in mRCC(e.g. CTLA-4, PD-1 antibodies) Slide 9a
So in conclusion for this trial, the tumor features did not predict for response, but we had a higher response than we expected higher than 20 years ago. This work continues and some of this work will educate us as we go into this new world of targeted immune therapy for kidney cancer.
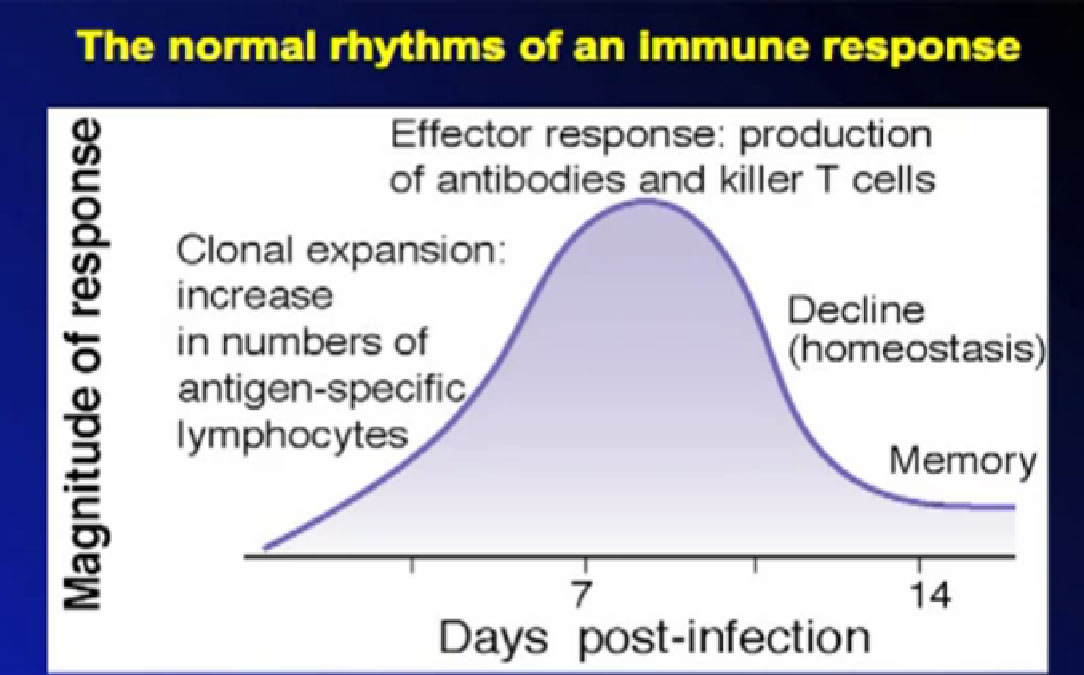 So as discussed, the immune system is not designed to fight cancer, but to fight infections. This graphic shows where the immune system turns on in response, and at its greatest, starts to shut off, as it is designed to do. The shut-off valves of the immune system–the brakes–are in some ways stronger than the gas pedal.
So as discussed, the immune system is not designed to fight cancer, but to fight infections. This graphic shows where the immune system turns on in response, and at its greatest, starts to shut off, as it is designed to do. The shut-off valves of the immune system–the brakes–are in some ways stronger than the gas pedal.
Releasing the Brakes
In the laboratory over the past ten years we have been able to identify what are those brakes are. Now we ask how to block those brakes. One of the most important brakes on the immune system is a protein called CTLA-4 (CytotoxicT-Lykmphoctye Antigen 4). We can block the action of this protein, to release one of the most important brakes to immune response to cancer.
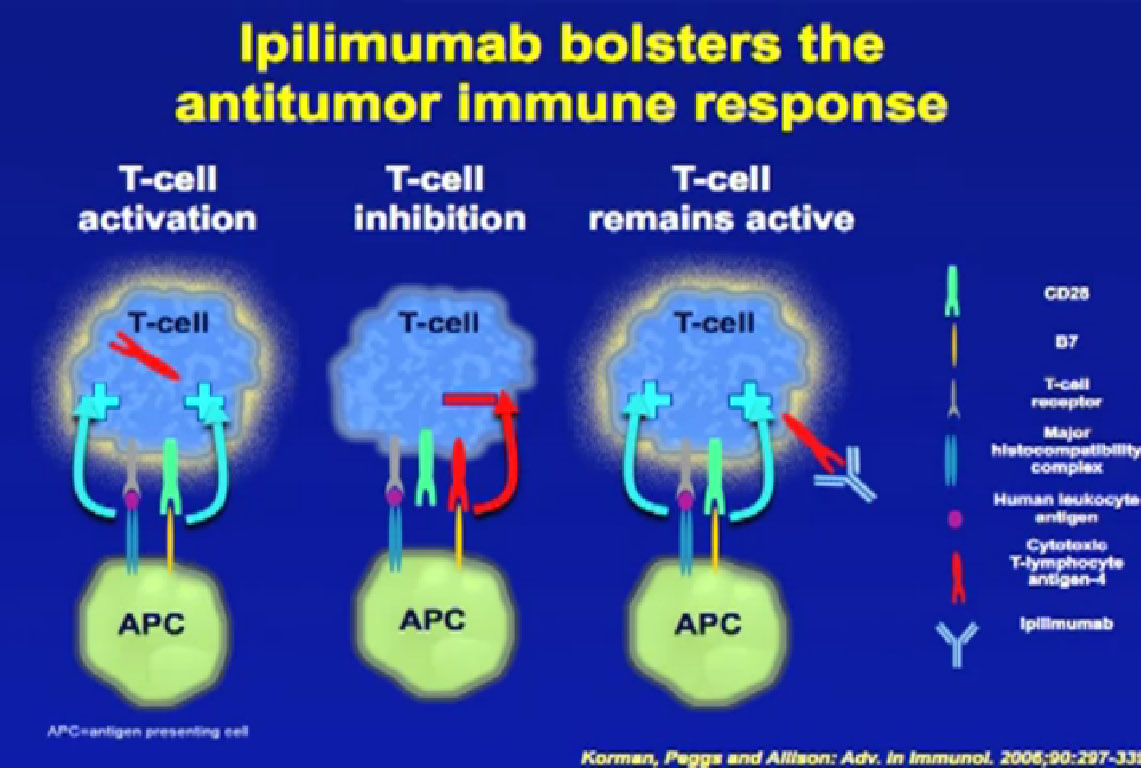 On this slide you see the T-cell on the left, an activated T cell, as it activates a protein, turns itself on. The same T–cell that turns itself on, puts out a protein on its surface, called CTLA-4. When that CTLA-4 protein comes in contact with another protein on that cell, an antigen-presenting cell, it actually shuts off the cell, a natural way of shutting off the immune response. That way these cells don’t overreact to an infection and attack healthy tissues, Now we can interrupt that interaction by bringing in a monoclonal antibody, which interrupts the connection between CTLA-4 and important proteins of the immune system. So we can block the natural shutoff that many patients experience.
On this slide you see the T-cell on the left, an activated T cell, as it activates a protein, turns itself on. The same T–cell that turns itself on, puts out a protein on its surface, called CTLA-4. When that CTLA-4 protein comes in contact with another protein on that cell, an antigen-presenting cell, it actually shuts off the cell, a natural way of shutting off the immune response. That way these cells don’t overreact to an infection and attack healthy tissues, Now we can interrupt that interaction by bringing in a monoclonal antibody, which interrupts the connection between CTLA-4 and important proteins of the immune system. So we can block the natural shutoff that many patients experience.
Does that work to clinical effect with kidney cancer patients? The short answer is it does. Data presented in the New England Journal of Medicine two years ago. Blocking this protein in melanoma gave improved outcomes, and this was an out-patient treatment, intravenous, given every three weeks for four doses. It’s actually been shown to improve survival for patients with metastatic melanoma.
Blocking CTLA-4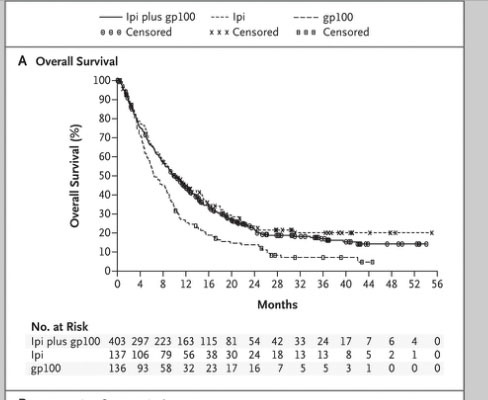
And it’s not treating the cancer; it’s treating the patient’s immune system. The immune system is going out and detecting the cancer in ways that it might not have before getting this antibody. It is outpatient therapy, but it is still associated with significant side effects. Most of those are auto-immune side effects, where the body is now being attacked by its own immune system. Some of these are been quite serious and patients have died from this treatment. We are getting smarter at managing the side effects so there are fewer major complications, but do not get the impression that it’s not serious treatment. It’s less toxic than IL2, can be given to more patients, but still has side effects.
Ipilimumab in mRCC
Single institution (NCI), Phase II trial
Major response rate of 9%.
Max dose test 3/mg/kg. (Dose response in melanoma)
Survival effect in melanoma despite low response rate.
Additional studies alone and in combination warranted. Slide 14
This data led to FDA approval last year of Ipilumimab, now called Yervoy. It was approved in melanoma, but it has been tried in kidney cancer patients at the National Cancer Institute in a very small 36 patients, phase II trial. The response rate, the chance that the tumor shrank by half, was only 9% in this trial. That may not sound great, it was tested at a dose which may not be the right dose; higher doses of treatment may be tested in the future. In melanoma we saw improvements in survival and saw lasting remissions for Stage IV patients. We saw those benefits even with a very small response rate. Go to the “Blocking CTLA-4” slide, and look way out on the survival curve towards the end. These are patients out 3 and 4 years, still in remission and off treatment. That happened even though the response rate to treatment wasn’t very high. It is possible that we might see a long-term benefit for some patients with kidney cancer with an agent like this. Some others are being tested in the coming year, so I would be thinking that additional studies with this approach are certainly warranted.
Steering the Body’s Immune System
We need not just the “accelerator” and the “brakes” to make these things work. We also need improved steering. Investigators want to improve steering by giving patients vaccine approaches. This may get patients’ immune systems to recognize proteins found on the tumor, to wake up and to go and attack those proteins, and to try to control the cancer. These approaches are now in phase III trials that have shown some encouraging results, where we will get some answers.
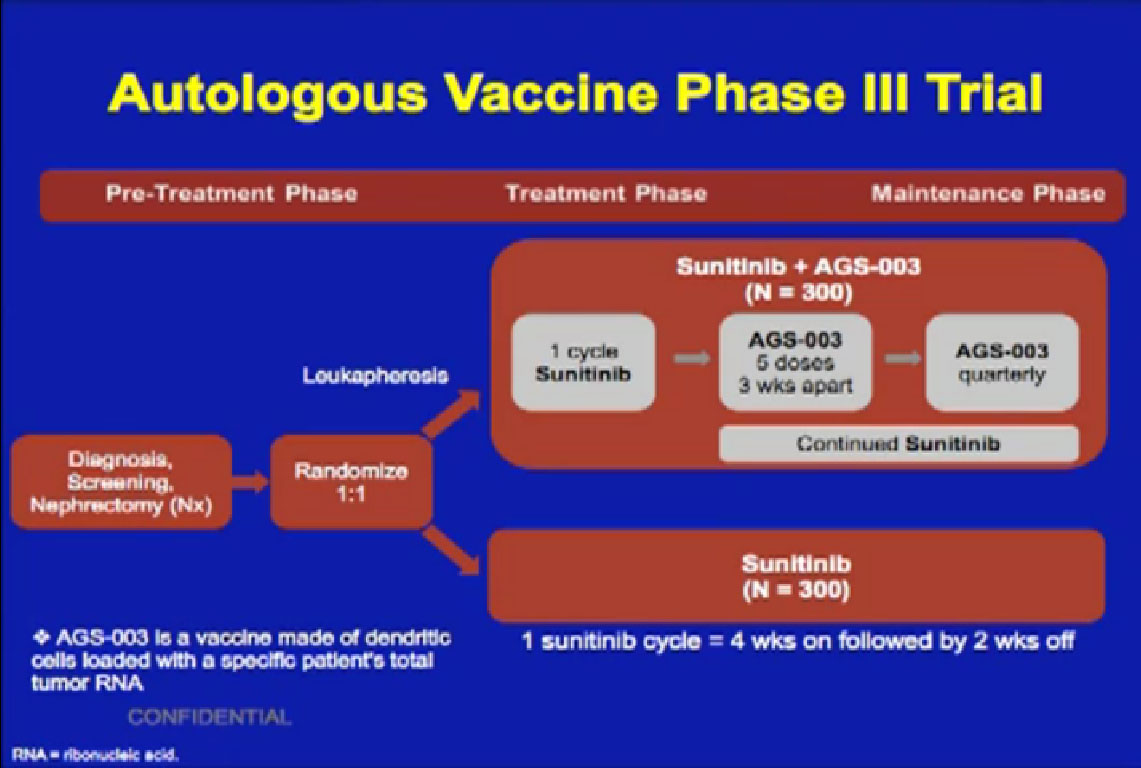 The agent AGS-003 is being combined with the most commonly used drug for kidney cancer, Sutent. Patients are randomized to use either Sutent alone or Sutent with this drug. Whether this will lead to more response rates and to more long term benefit than with Sutent alone remains to be seen. At least we will get an answer from a large Phase III trial. One of the problems with immunotherapy trials over the years is that they haven’t been large enough to give us clear answers. But this trial is enrolling patients and there are others like it.
The agent AGS-003 is being combined with the most commonly used drug for kidney cancer, Sutent. Patients are randomized to use either Sutent alone or Sutent with this drug. Whether this will lead to more response rates and to more long term benefit than with Sutent alone remains to be seen. At least we will get an answer from a large Phase III trial. One of the problems with immunotherapy trials over the years is that they haven’t been large enough to give us clear answers. But this trial is enrolling patients and there are others like it.
Putting up the Barbed Wire Defense
Since we’re in Texas, so I thought I’d give you a barbed wire analogy. One way in which tumor cells evade detection by the immune system putting up this “barbed wire” on their surfaces. When a T-cell comes, tries to sniff out the cancer cell, it is shut down, as opposed to going on to kill the tumor cell. We now know a little bit about that natural barbed wire which comes into play with this particularly important protein interaction, the programmed death pathway, and what that stands for is the inactivation, or the programmed death of the T-cell, which acts like barbed wire.
Why is this important? In kidney cancer, many patients’ tumors are coated with this barbed wire. Patients who have this on their initial tumor specimen have been shown to be more likely to have more disease recurrence in the future. Since we can identify the barbed wire, can we block its activity and lead to an improved effect? The question is, can we target this protein and can we improve outcomes? There is early evidence that maybe we can. This is a Phase I trial, admittedly very early.
Programmed Death (PD)-1/PD-L1 Pathway: The Basics
Several tumor types, including RCC, have been shown to over express inducible PD-L1
Over expression of PD-L1 by RCC tumors has been shown to be associated with adverse clinical/pathologic features, including the following:
More aggressive disease
Shorter survival
Also report to impair tumor immunity
Can PD-1 pathway blockage head to clinical benefit? Slide 18
Phase I Study to Evaluate the Safety and Antitumor Activity of Biweekly BMS-936558 (Anti-PD-1, MDX-1106/ONO-4538) in Patients with
Renal Cell Carcinoma and Other Advanced Refractory Malignancies.
Patients received an outpatient treatment, intravenously, with a drug that doesn’t even have a name yet, BMS-936558, a PD-1 anti-body. This is blocking the interaction between the barbed wire and T-cells. In this trial it is given every two weeks; on other trials it’s been given every three weeks. Patients were allowed to stay on this drug for up to two years as long as they were getting some clinical benefit.
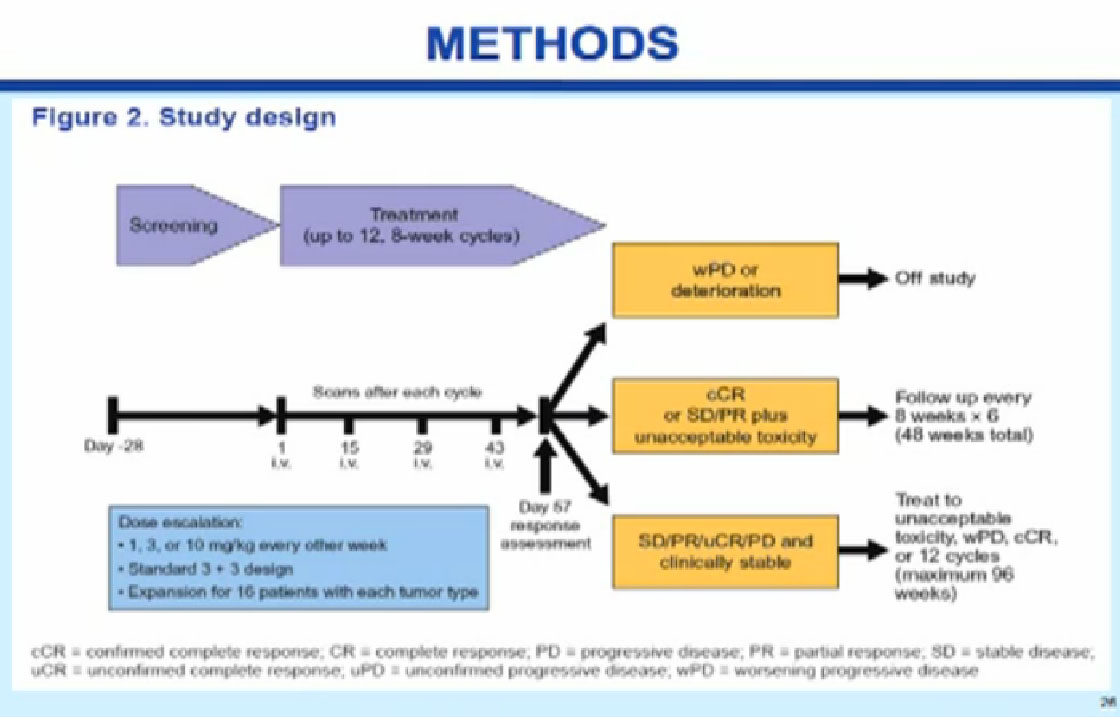
SAFETY RESULTS; ALL PATIENTS
For the entire group, MTD (Maximum toxic dose) was not reached at doses of 1, 3, and 10 mg/kg
There was no apparent relationship between drug dose & AE (adverse effects) frequency
One treatment-related death
Grade 4 drug-related pneumonitis Slide 20
The main reason to do a phase I trial is to test the safety of the drug. This drug was found to be relatively safe, and we got up to the highest dose without getting into serious side effects. There didn’t seem to be a relationship between the dose and the side effects, which was good. There were still 10-15% patients who had serious side effects. In this group of kidney cancer patients and there was one treatment-related death, due to an inflammation in the lung that was probably contributed to by the drug.
SAFETY RESULTS; ALL PATIENTS
Any grade, drug-related (investigator attributed*) serious AEs (SAEs) occurring at a frequency >1% in the entire study group (n=126)
Total with an SAE=11%
Investigations=3.2%
Endocrine disorders=2.4%
General disorders and administration site conditions=1.6%
Hepatobiliary disorder=1.6%
Neoplasms benign, malignant and unspecified=1.6%
Respiratory, thoracic and mediastinal disorders=1.6% Slide 20a
This trial of 126 patients was a much larger group and not just a kidney cancer trial with five cancer types in this trial. There are now 300 patients in this trial, and we’ll get more data in the next several months. But in general the safety population is much larger than the efficacy population. The main point here is that serious adverse events occur in about 11% of all patients, much less than in HD IL2 and CTLA-4.
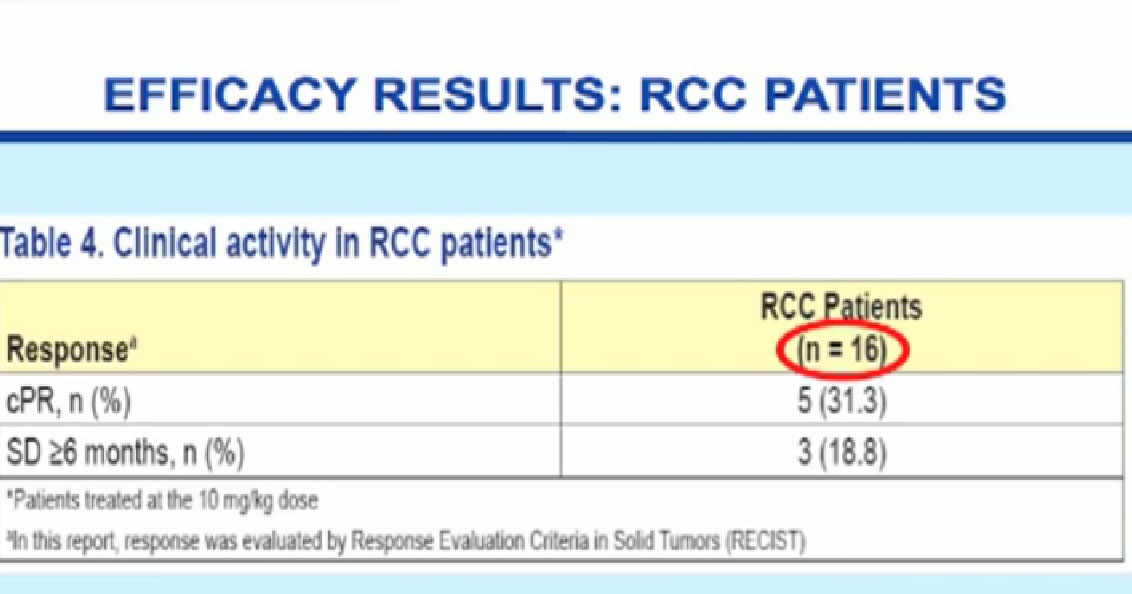
What did we see in terms of effectiveness? For the first 16 patients analyzed, we saw five partial responses (31.3%), and three patients (18.8%) with stability that lasted over six months. About 50 % of patients were getting some clinical benefit with this antibody. Once again, a small number of patients tested.
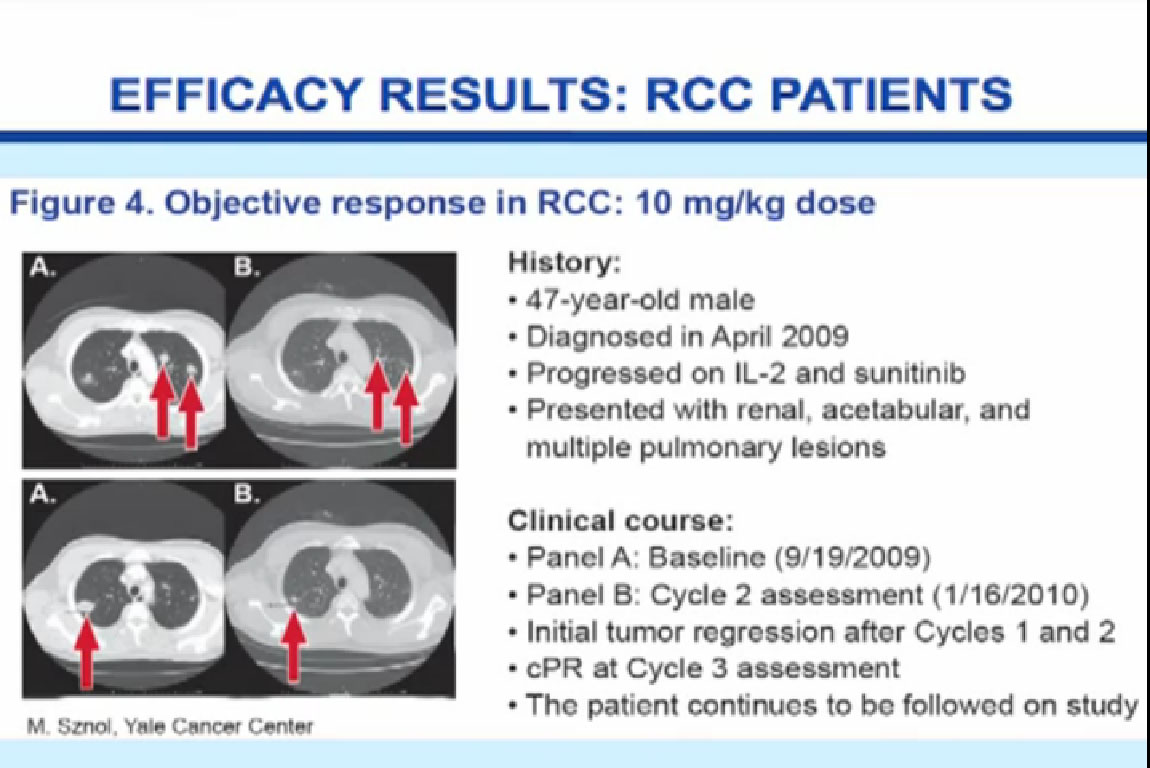
Here is an example of the patients we studied, a young man who was diagnosed in 2009, received IL2 and progressed, received Sutent and progressed, had disease in multiple places, including the kidney and the bones and lungs. You can see the before and after pictures after receiving two courses of treatment of ipilumimab with a significant response in the lungs, which is ongoing therapy after two years.
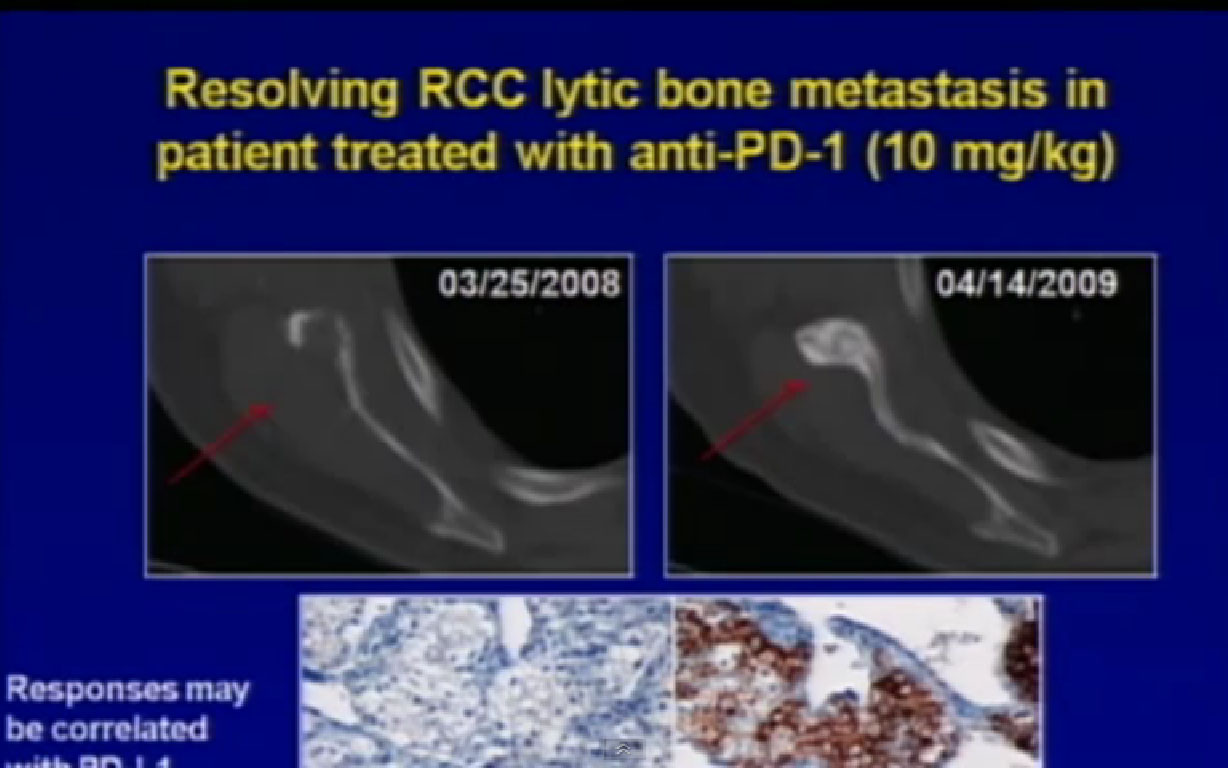 We’ve also seen responses in some unusual places. One place that a lot of our VEGF targeted patients fail is in the bone. This is a patient, before and after, who had a response with a bone metastases you can see here (left scan), and actual healing bone (right scan), just a year later with treatment. That’s certainly an encouraging result for us.
We’ve also seen responses in some unusual places. One place that a lot of our VEGF targeted patients fail is in the bone. This is a patient, before and after, who had a response with a bone metastases you can see here (left scan), and actual healing bone (right scan), just a year later with treatment. That’s certainly an encouraging result for us.
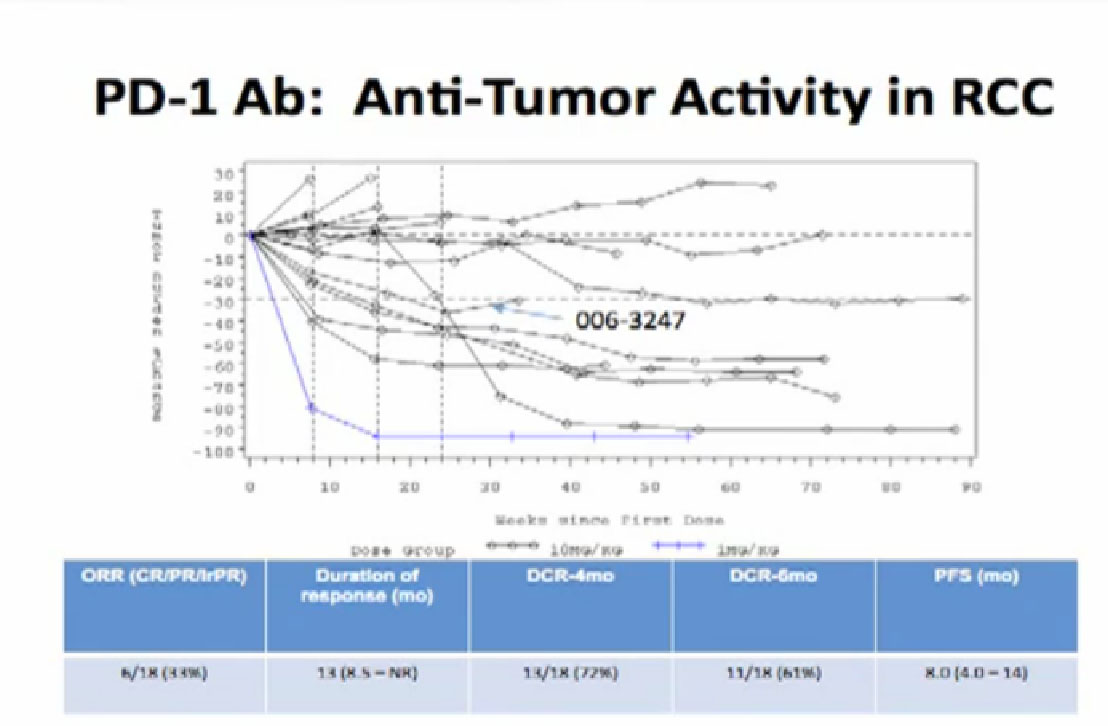
How long do these responses last?
For this admittedly small group, it looks like the major responses for a majority of these patients who had a response, that response lasted over a year. Most who had a response still have that response at a year. Whether that response will continue or stop, when the drug is stopped, remains to be seen. That will be a very important question. But the good news is the patients can tolerate this treatment for a year or more.
In summary, the PD-1 antibody concept is very early, but it has displayed reasonable side effects at all doses we’ve tested, and we seen some anti-tumor activity in some patients with kidney cancer.
PD-1 Antibody Summary
At this EARLY point, BMS 936558 has displayed manageable side effect at all dose levels tested
Anti-tumor activity observed in a small number of patients with RCC
Responses may be durable?
16 patients added to this cohort-ASCO 2012
Responses in NSCLC
Trials launched in 2011 in RCC:
Dose finding Phase II study in patients with prior therapy
Biomarker Trial
Combination RX + VEGF TKI (Phase I)
Were these remissions, i.e., durable, or just responses? Will the patients’ tumors grow after we stop the drug? We don’t know that yet, but we’ve added 16 more patients to the study. We will present data at ASCO in two months (6/12), with more information on this anti-body soon. It is important to note that with this drug and for the patients on it and for the field of immunotherapy for cancer that we are we seeing responses for kidney cancer and melanoma, where we’ve seen immune responses before. It is quite important also that we are also seeing responses in lung cancer. Should that be true, that will bring a lot of energy into the field going forward, as lung cancer is obviously a much more common illness, with very few options available.
There were a lot of new trials launched in 2011. We’ll have a Phase II trial in 2012, including multiple doses, to find the best dose in this treatment and also in combinations with other drugs like Sunitinib to find the best combinations.
So for those who say, “Is there a role for immunotherapy for RCC?” I would say there’s been a lot of progress over the last 30 years in the field of immunology, and we can now better target immunotherapy, and it’s worth preserving.
Is there a role for immunotherapy for mRCC? There has been progress in the field of immunology in the last 30 years
Immunotherapy can be “targeted”
Immunotherapy is worth preserving Slide 25
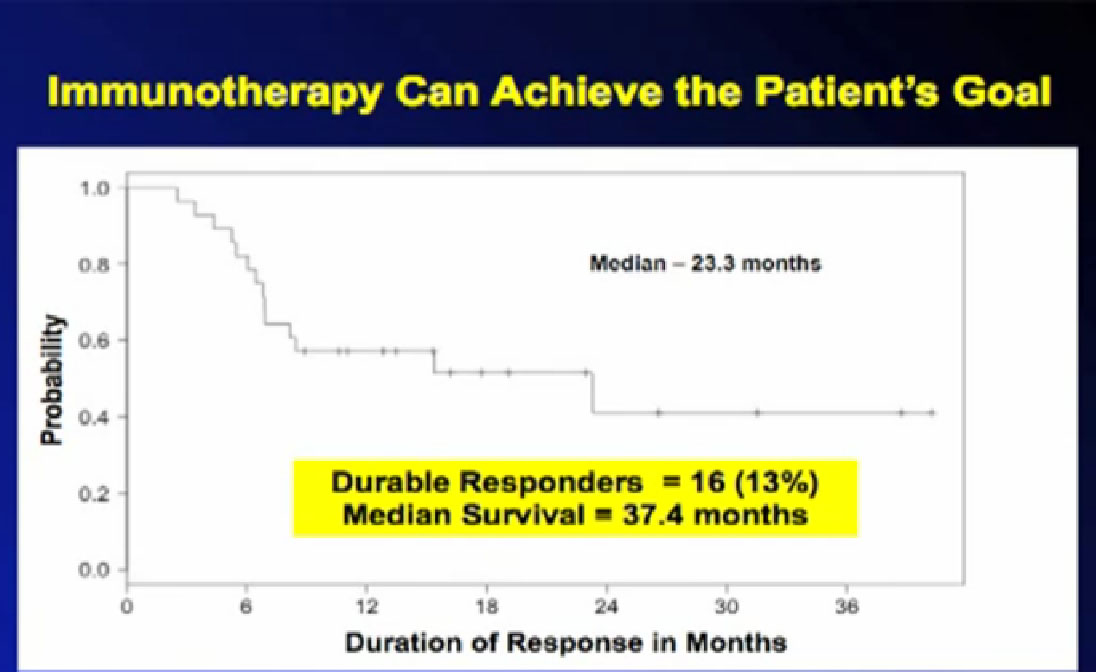
There is a role for immunotherapies shown by survival curves like this; this is from the SELECT trial mentioned before. These are patients who responded to treatment, now out three years, off treatment. The 13% percent of these patients, 16 patients have that benefit which really achieves the patient’s goal, which is worthwhile to try to get for more patients. It is proof of principal that we can do this, but we need to do it for lots more patients. If we work on this, hopefully we can achieve Dr. Wood’s goal of more cures for kidney cancer, with targeted immunotherapy.
QED
Audience: Does the pathway of the targeted drug therapy have any impact on whether or not immunotherapy would have any effect on tumor growth or not? Different targeted therapies have different modes of blocking pathways. Would that have impact, or has your research shown any impact on different pathways.
Mc Dermott: “That is a very good question and not simple to answer. Many of the treatments I talked about, like the cytokines, like IL2 or interferon or CTLA-4 work on the patient’s immune system-we think ONLY, only, meaning they are boosting the immune system to an established cancer. For reasons we don’t completely understand, that is effective in some patients and not in others. In the case of the PD-1 antibodies, they may be acting on the immune system, and may act on the tumor as well. There is some evidence of that in the early research, as I mentioned with patients who have this barbed wire on the surface of their tumors, or have this pathway activated, or when this pathway is on, they may be more likely to respond to this approach. Meaning, when the barbwire is up, and you can block its activity, maybe you can lead to an effective immune response. And what that may say something important about the pathway, but it may also say something about the immune system’s response. The barbed wire, we think, is up, as a reaction, a survival mechanism for some tumors. That is one of the reasons that cancer is so difficult to deal with, that there are so many different ways the tumor can evade the treatments. That barbed wire, that PD-1 may be one of those protection mechanisms. So that means tumor turns on, the barbed wire is up when the immune system is there, you can’t kill it, but you can block the barbed wire, take these immune cells are right there and it can kill the cancer. This may explain why some patients have a benefit and some don’t. And we are now looking to see, if your tumor has barbed wire, whether you are more likely to respond to treatment. There will be a lot more information about that in the future. Good Question.”
Question: I wonder if you knew if there was a clinical trial going on for prostate cancer, believe it is called XPl 4(sic), and its for bone mets, and if you knew anything about it’s work with RCC.
McDermott: “Re this med and RCC,I don’t treat prostate cancer, just melanoma and kidney cancer. Is the drug you are talking about XL 184? This is a drug that is in early trials, and maybe Dr. Jonasch will talk about it and similar drugs. These drugs work in the blood vessels to attack the cancer. They target two proteins, VEGF, which you’ll hear a lot about, and another protein which is important, C-MET, called MET inhibitors. There has been interesting activity in prostate cancer with this drug in trial, and a very small trial we participated in Boston with this agent with kidney cancer patients. You will see more info on this drug at the ASCO meeting in June and the info will be public on May 16. ASCO publishes all its info, so you will be able to get your hands on the results from that very small trial in a very few weeks, which is good. It’s a drug that has promise, but it has some side effects that need to be managed. The fundamental question here is for the kidney cancer advocacy community, is it going to be tested in kidney cancer? We are still not definite yet, but we are pushing to test this class of drugs in kidney cancer, this particular drug. We are pushing sponsors to do trials, but they are most interested in prostate cancer. When those trials are to start and with kidney cancer, we are not entirely clear. We need to push them to keep developing these interesting drugs for our patients.”
ME: This is an extension of the question, as to how you choose the best patients who have the best chance of responding to IL2. Does the histology or the pathology, and frankly I don’t know the difference between the two, have a major impact, and can you expand on that?
McDermott: “Yes, I may have gone too quickly on that. We thought the answer was yes, and when it comes to big differences, clear cell versus non=clear cell, it does, meaning when patients come to us, non-clear cell cancer for IL2, we will say to them, ‘It’s not going to help you, or the chances are so small, that the harm or potential harm outweighs the help.’ So to answer one of your questions, non-clear cell histology, which is simply a medical term for ‘What does this thing look like under the microscope?’.
For non-clear cell histologies, we don’t offer IL2. Whether one of these newer immune therapies work for that class of patients, should be tested because there is hope. That is why I mention the lung cancer story, because if it can work in lung cancer, it might in one of these less common kidney cancer types. It may work for many other forms of cancer. So that is a hopeful thing and exciting. But to take it a step further, while we thought clear cell cancer was more likely to benefit, we also thought it was that certain types of clear cell cancer were likely to benefit. That is what we tried to prove in that trial, but we couldn’t prove it. Doesn’t mean it wasn’t so, we just couldn’t prove it. We haven’t improved out selection ability based on looking under the microscope in ways that we had hoped. We can do it a little bit, and that’s why the response rate went from 14% when the drug was approved to 25 % today, but it’s not enough, so patients don’t have to go through the side effects if they are not to benefit from the treatment.”
Question; I don’t know if I know enough to ask a coherent question, but this is related. Why would it be that certain tumor types or subtypes would respond to IL2 or not? Is it because their profile of cell surface markers that let the immune system targets them better?
McDermott: “That’s actually a very sophisticated question, and one that does not have a clear answer. It may be, one thing you mentioned, it may be that the immune system usually recognized proteins, usually proteins associated with infections like viruses or bacteria. So it may be, as you point, that there are certain tumors that have show these proteins to the immune system better than others. And then the immune system can detect these proteins and say, “This is something wrong here, let’s go kill it”. That’s one possibility, and we tried to prove that in our trial, because one of the proteins we looked at was the protein called carbonic anhydrase IX. We thought this protein was being recognized by the immune system and that might predict for benefit, but we couldn’t prove it. So many people suggested your theory, which was that these proteins are important, but we could not prove it in this trial. It doesn’t mean that there aren’t others that are important, we just couldn’t confirm it. It may also mean that there are certain aspects of the tumor, that even though it is recognized by the tumor, something is preventing it from dying under the strain of an immune attack. That has to do with how well a cancer cell dies under stress. There may be some that don’t automatically shut down when they are being surrounded, that’s part of it. There are also other important proteins that the tumor can make to avoid detection so the opposite of what you are talking about, that barbed wire analogy. These are proteins that the tumor can turn on, and act like barbed wire, so that when the cells are coming it, they are turned off. So there’s a variety of different theories and more than one aspect of that. It’s a fairly complicated story.”
Me; Back to CA IX, the fact that you expected that more of that would lead to greater response, you found the opposite to be true?
“No, we didn’t find the opposite to be true, we found that the folks who had CA IX protein on their tumors did well, or better than the old numbers. Those patients responded at about the 25%, but they did as good anyone. What was different was that the patients who didn’t have CA IX on their tumor did just as well. So that is why I said it was bad for the research, but good for those patients. We would have assumed, if we hadn’t done the trial, that to those patients, we might have said, ‘You don’t have CA IX on your tumor, no IL2.’ We did the trial to show that, we couldn’t. That means that going forward,, we should include those patients getting IL2.”
Me: I went on IL2, and I am a complete responder, and I had a relatively low level of CA IX, so I always watch that and wonder.
McDermott: “You are an example of why my research career is floundering,” laughing.
Me; Sorry, but it works for me, works for me!
McDermott: “I am very happy for you and for those patients on the trial. That is why you do the research. The CA IX story, and this was scary when I saw it, actually made it into the medical training literature. So if you were an oncology fellow, you learned that McDermott at Beth Israel and others said CA IX was important, and that high CA IX patients should IL2 .And then when I saw the results of the trial, I thought, O my gosh, this has become gospel, and is maybe not right. You are a perfect example of it, and it means we need to do more research into the area . You are an excellent plant could not have been better.”
Audience: Did you say that you have some non-clear cell patients on the MDX trials?
“Not yet. Most of them whose tumor type we knew all have been clear cell. But in some of the future trials there are small numbers of non-clear cell patients who will be enrolled. If there is any sense of activity, it may encourage study sponsors/drug companies to develop it in non- clear cell patients. They will not be excluded on trials going forward.”
Audience: So you your knowledge there is not one which is limited (to non-clear cell) for MDX 186?
“I haven’t seen a response in that category, since most of the patients are clear cell the assumption in the field is that clear cell benefits from immunotherapy, so when you try to prove a principal, they try to collect the patients most likely to benefit, so not to put people through treatment that will not benefit. But now that we have this proven—really not proven—but a hint of effectiveness, different types of patients are being included in the trial. For example, the trial which includes the addition of MDX agent with Sutent allows non-clear cell patients. Hopefully there will be more of those in the future. The good news is there is more than one drug company developing these antibodies, and I will include those in my talk this afternoon.
We don’t do all the work at our place, but I haven’t seen a non-clear cell responder at my place. I have seen responses with very aggressive tumors, that had sarcomatoid features, for example, but I haven’t seen one with non-clear cell. That does not mean that it has not occurred.”














