Dr. Christopher Wood
Integration of Surgery and Systemic Therapy in the Treatment of Kidney Cancer
Also referred to by the KCA as “Role of Cytoreductive Surgery in the Treatment of Metastatic RCC
UT MD Anderson Cancer Center
KCA National Patient Conference: April 14, 2012
 The topic “Integration of Surgery and Systemic Therapy in the Treatment of Kidney Cancer” is near to my heart and in active research at MD A
The topic “Integration of Surgery and Systemic Therapy in the Treatment of Kidney Cancer” is near to my heart and in active research at MD A
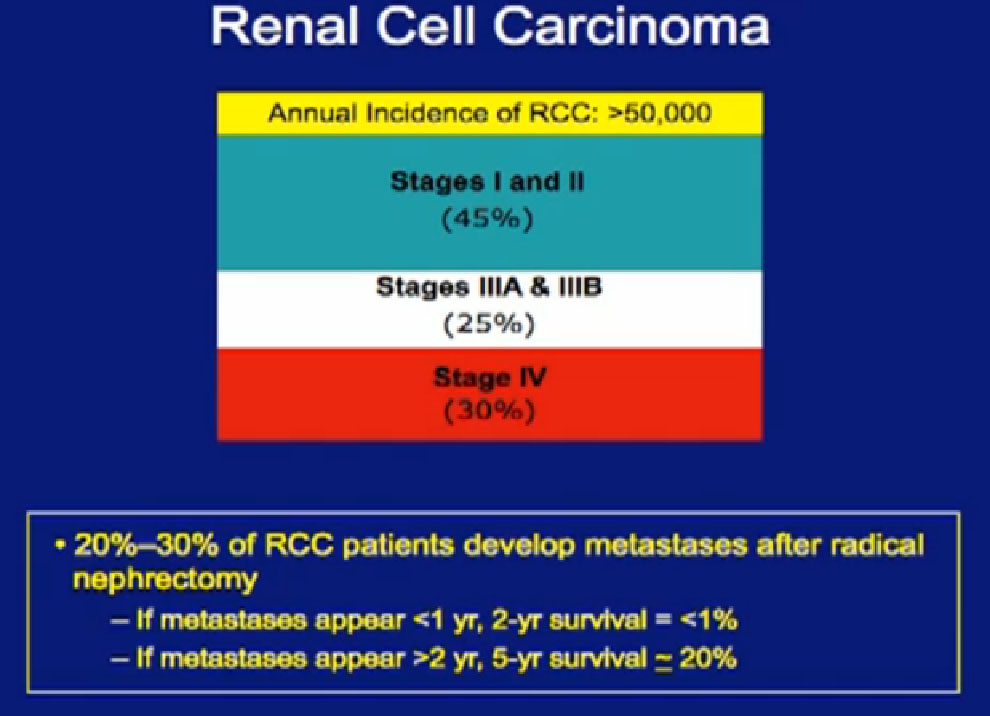
nderson Cancer Center. The vast majority of patients present to us with locally advanced disease, and the reality is that almost half of patients with kidney cancer will at some time develop metastatic disease, which we all know is currently not curable.
Again, as to stage, the vast majority of patients present to us with locally advanced disease, and the reality is that almost half of patients with kidney cancer will at some time develop metastatic disease, which we all know is currently not curable. (Editor’s note: my stage IV RCC with innumerable lung mets in 2004 is now in remission or “cured”, or close enough to “cured”, that I must add this note. It is still shocking to understand that Stage IV RCC is considered incurable. PZ)
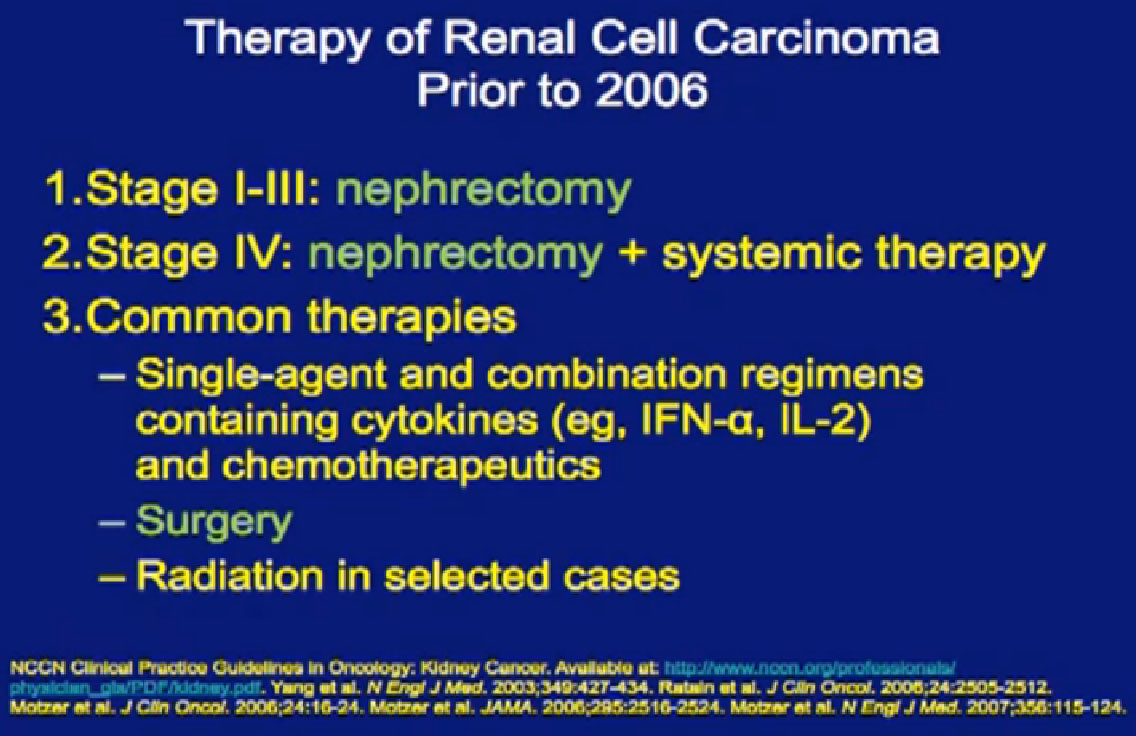
Six years ago, we did not have much to talk about. I could show this slide and sit down. For locally advanced disease, we took out the kidney; for stage IV disease, we took out the kidney and gave systemic treatment, usually cytokines.
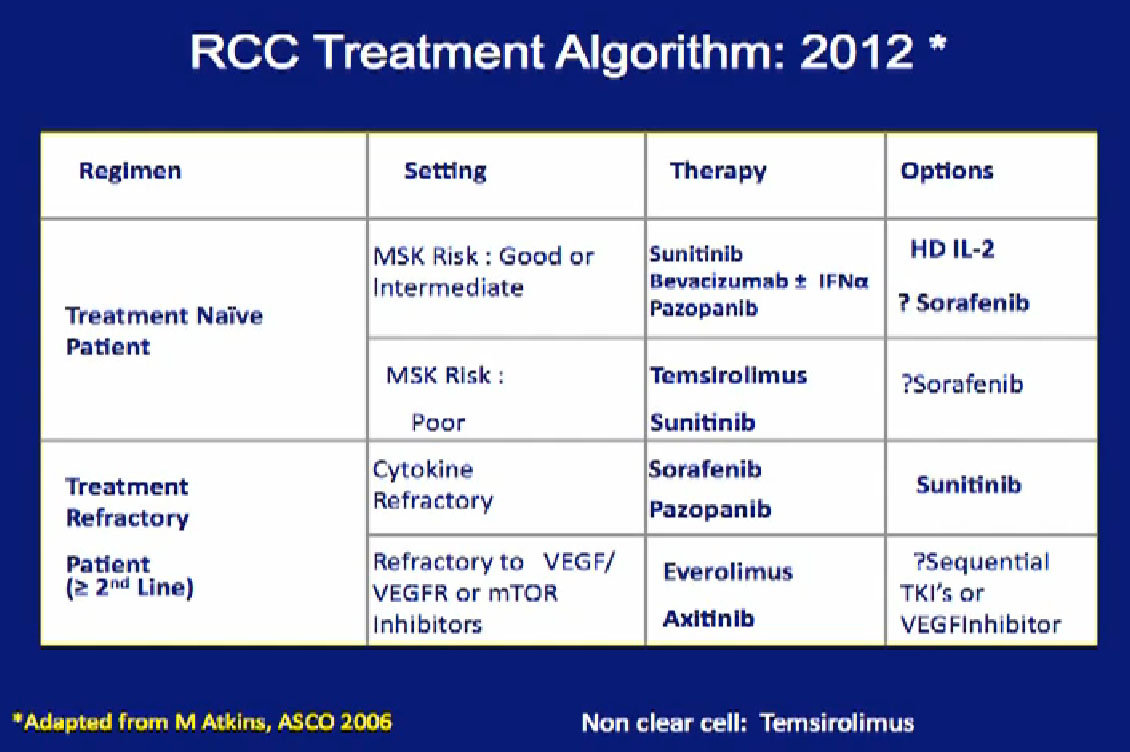
Now we almost have too many therapies and don’t know how to use them all. How do we integrate surgery into the context of these treatments that we offer patients with metastatic disease? The sad reailties of this are no home runs. These therapies control the disease for some time in these molecular pathways they target. But in most patients, resistance will develop an d they have to move on to the next treatment.
d they have to move on to the next treatment.
I will talk about how we integrate surgery into systemic treatment in 2012, the role of cytoreductive surgery, and then introduce the idea of pre-surgical therapy, something that we have been studying here at MD Anderson that has shown some promise.
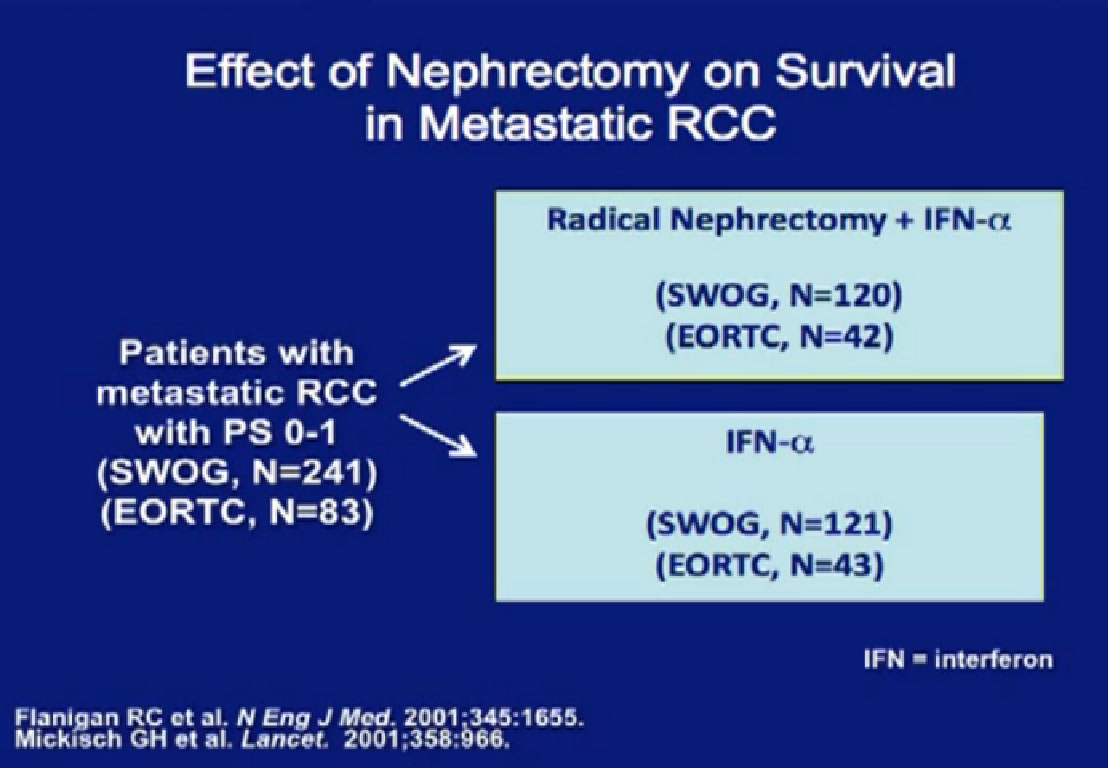 These are two randomized trials, one in the US, the other in Europe, which demonstrate the benefit of patients undergoing cytoreductive surgery. The first is the EORTC trial where patients were randomized to upfront surgery fo
These are two randomized trials, one in the US, the other in Europe, which demonstrate the benefit of patients undergoing cytoreductive surgery. The first is the EORTC trial where patients were randomized to upfront surgery fo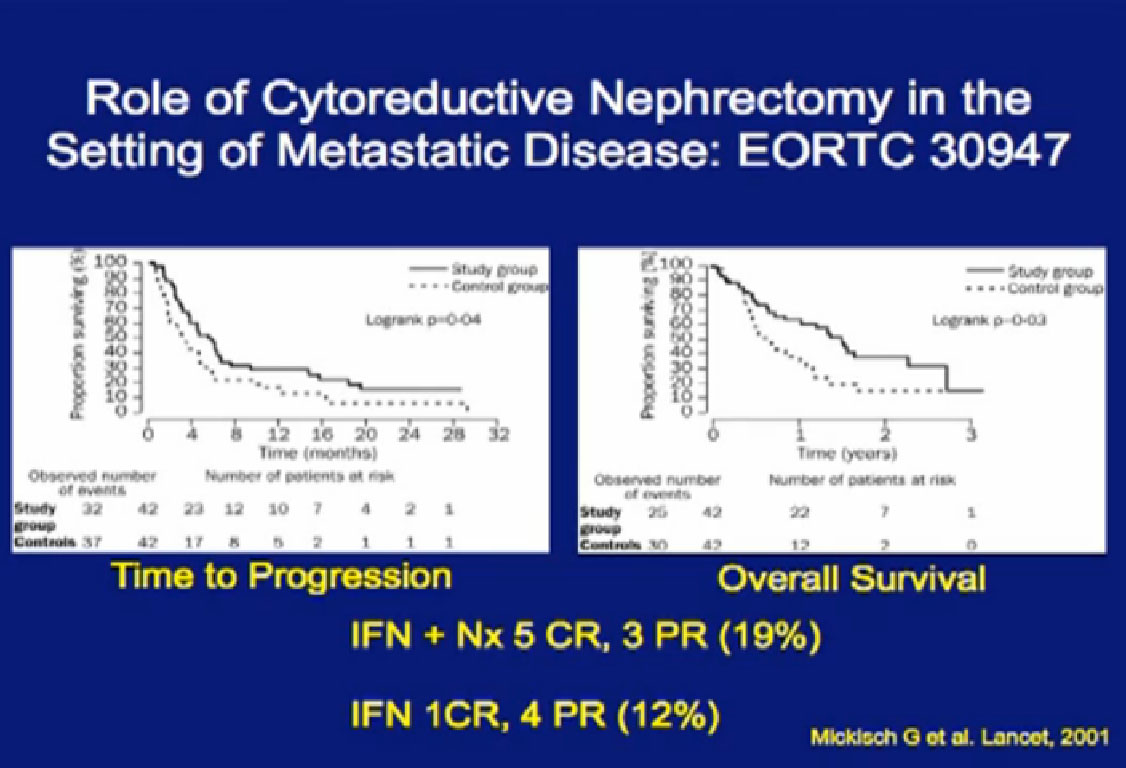 llowed by interferon, or by interferon alone.
llowed by interferon, or by interferon alone.
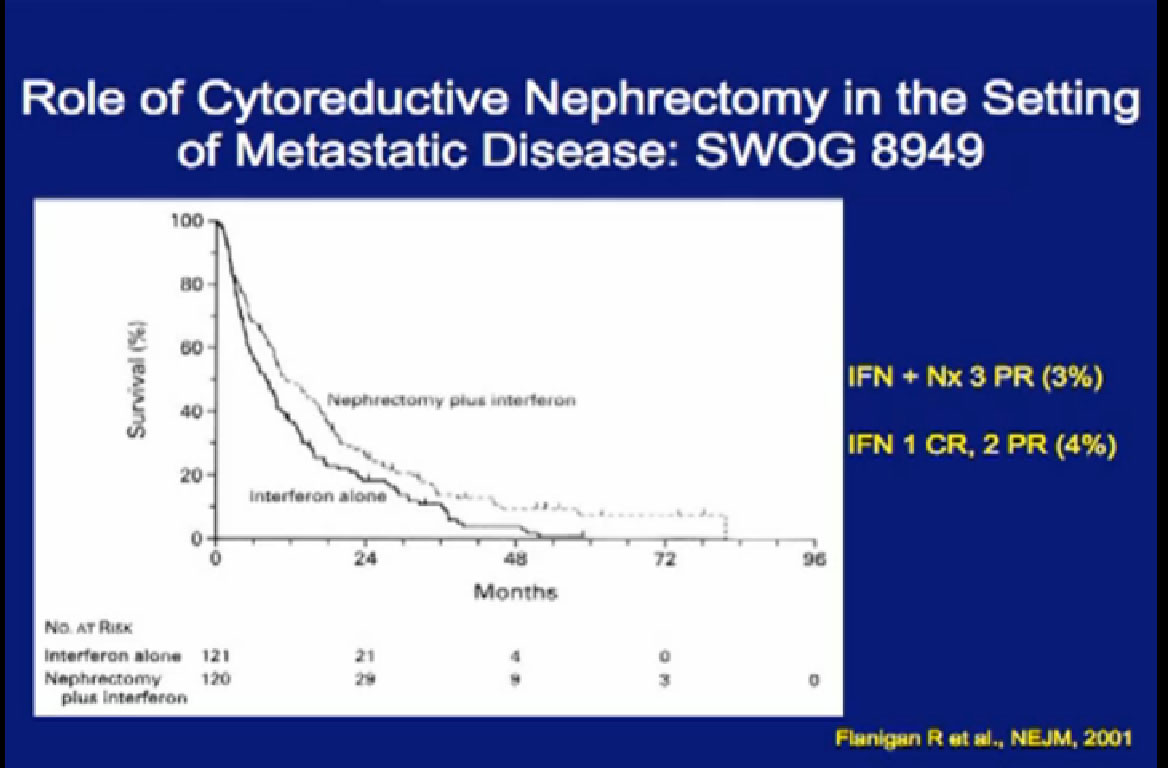
Next is the SWOG (South West Oncology Group) trial done in the US, again demonstrating the value of undergoing a nephrectomy prior to interferon, versus interferon alone? Frankly, many surgeons referred to this trial as “Surgery followed by ineffective therapy is better than ineffective therapy alone.” You can see by these that the response was only 3-4%.
Now that we have more effective therapy, what is the role of surgery in the context of patients with metastatic disease? We don’t even know why cytoreductive surgery works. Does it just reduce tumor burden? Does it produce some sort of immunologic phenomenon where tumor antigens are exposed or is there an immunological “sink”. Is it an alteration in the metabolic milieu where taking out someone’s kidney and altering the ph in the body somehow is anti-tumoral? Or is taking out the “mother ship”, where some sort of endocrine or paracrine phenomenon that promotes metastases. We have absolutely no idea how it works.
Why not take out everybody’s kidney in the setting of metastatic disease? 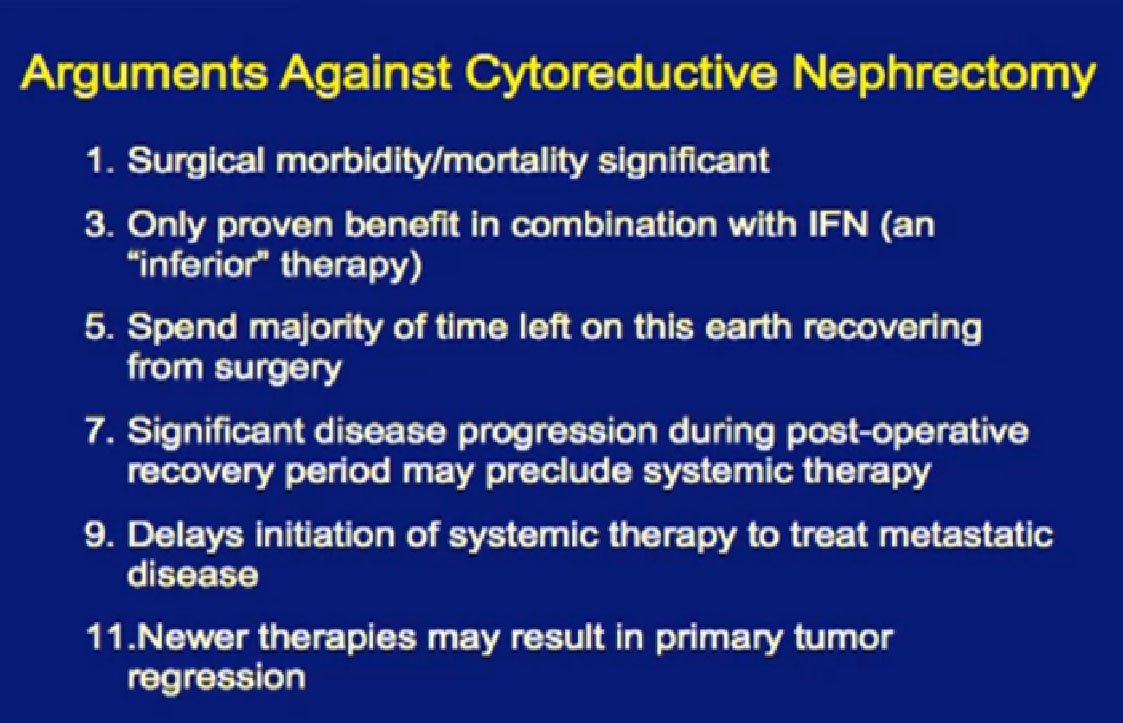
The morbidity and side effects can be significant, and people can die from this operation. Though it has been proven beneficial in the context of interferon, and it’s quite possible that patients will spend the majority of their time left on earth recovering from surgery. We’ve seen metastatic disease explode post-operatively. Those patients never even get to go on to targeted therapy after surgery. Perhaps these new therapies will cause the primary tumor to shrink. Maybe we don’t need to take out everyone’s kidneys in the face of metastatic disease.
The French are testing this in their Carmena 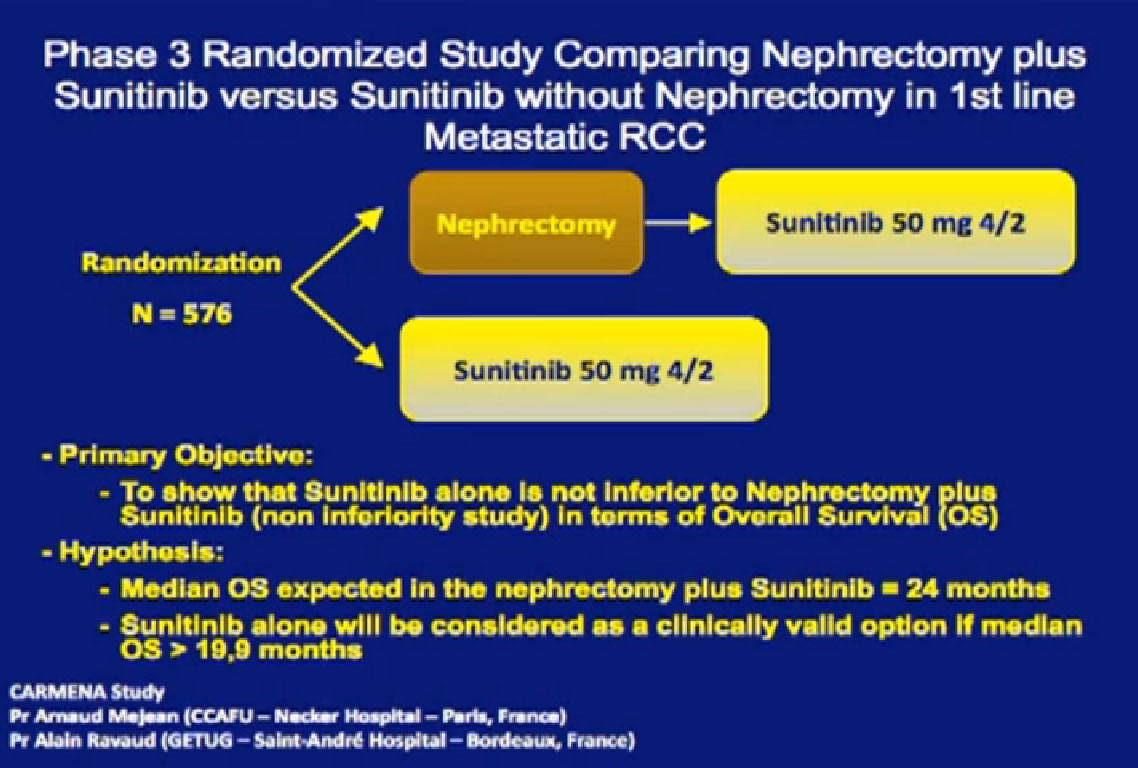 trial. Patients are randomized to an upfront nephrectomy followed by Sunitinib versus Sunitinib alone. It is a non inferiority study design, and the randomization is 576 patients. There is a problem; the trial is not at all accruing very well. Patients don’t like to have surgery randomized, with a computer saying, “You’re going to have surgery, and you are not”.
trial. Patients are randomized to an upfront nephrectomy followed by Sunitinib versus Sunitinib alone. It is a non inferiority study design, and the randomization is 576 patients. There is a problem; the trial is not at all accruing very well. Patients don’t like to have surgery randomized, with a computer saying, “You’re going to have surgery, and you are not”.
With the slow accrual to this trial it will be many years before we have an answer. Sunitinib may not even be relevant at that time. And what are we doing for patients in the meantime? Should I take the kidney out or not?
There is evidence that removing the kid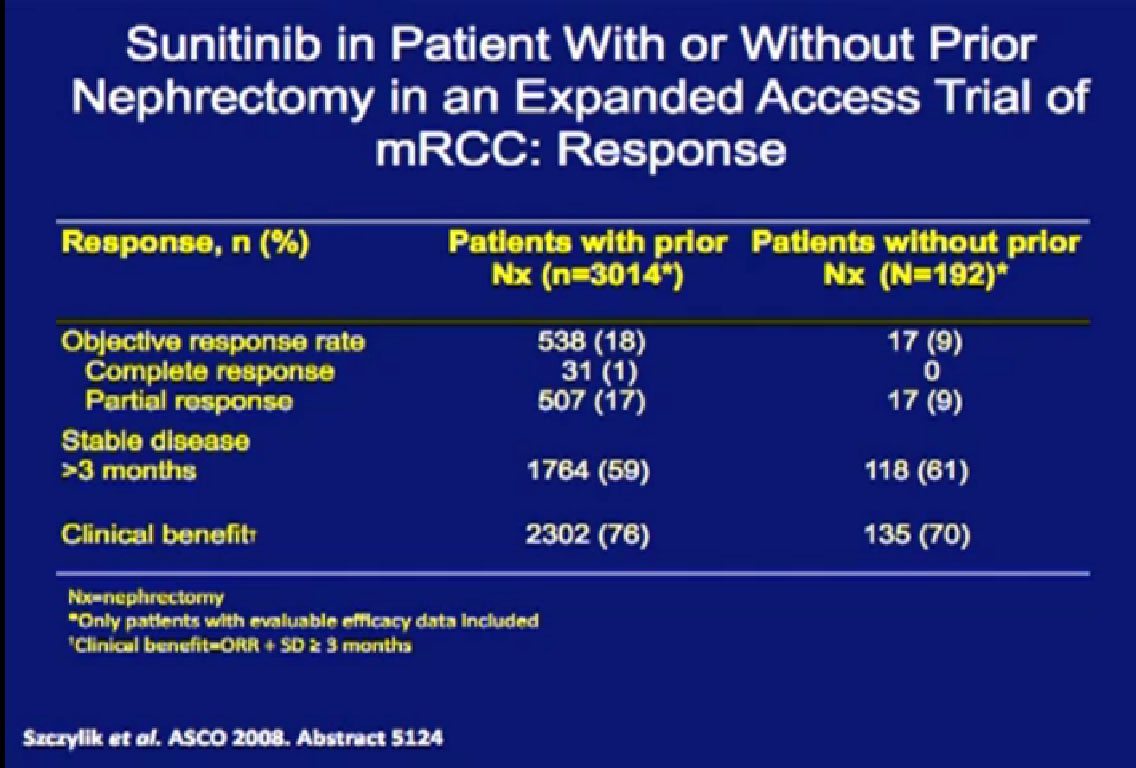 ney in the presence of metastatic disease is beneficial, from retrospective data derived from the expanded access trial with Sunitinib. These patients had their
ney in the presence of metastatic disease is beneficial, from retrospective data derived from the expanded access trial with Sunitinib. These patients had their
kidney removed, not just cytoreductive surgery, but any time in their past.
They were compared to patients treated with their kidney in place. 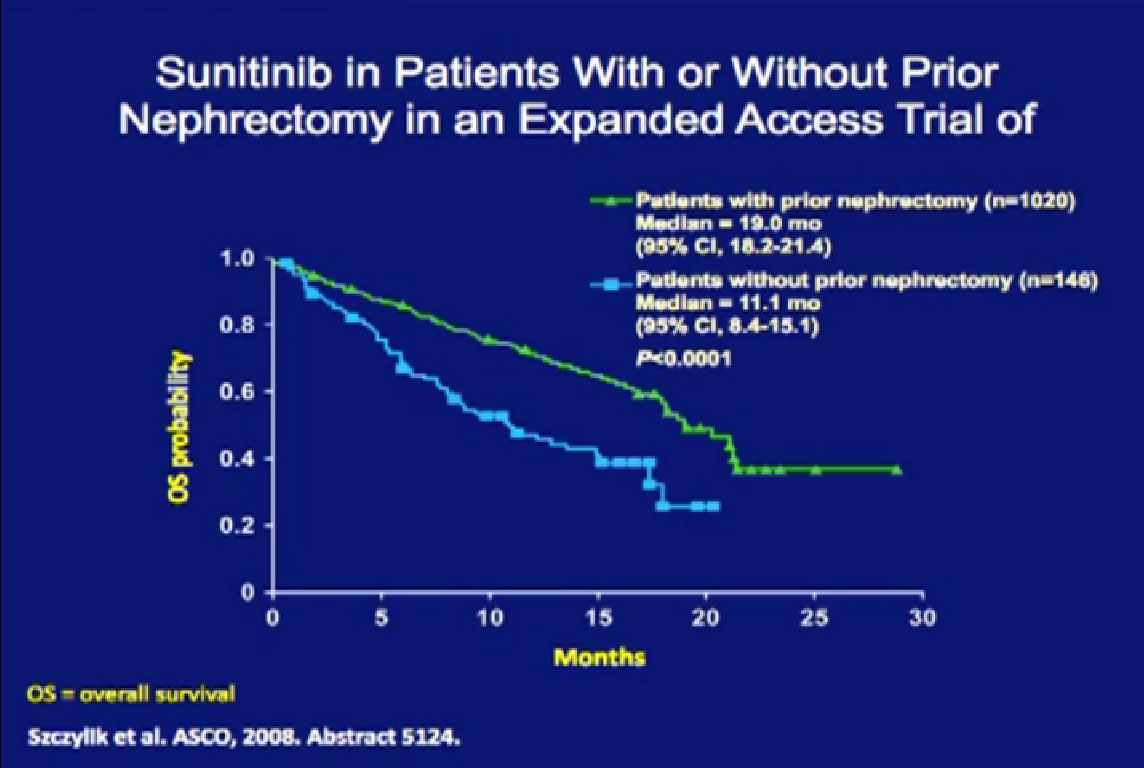 Patients who had their tumor out had a much better response rate than those treated with their tumors in place.
Patients who had their tumor out had a much better response rate than those treated with their tumors in place. 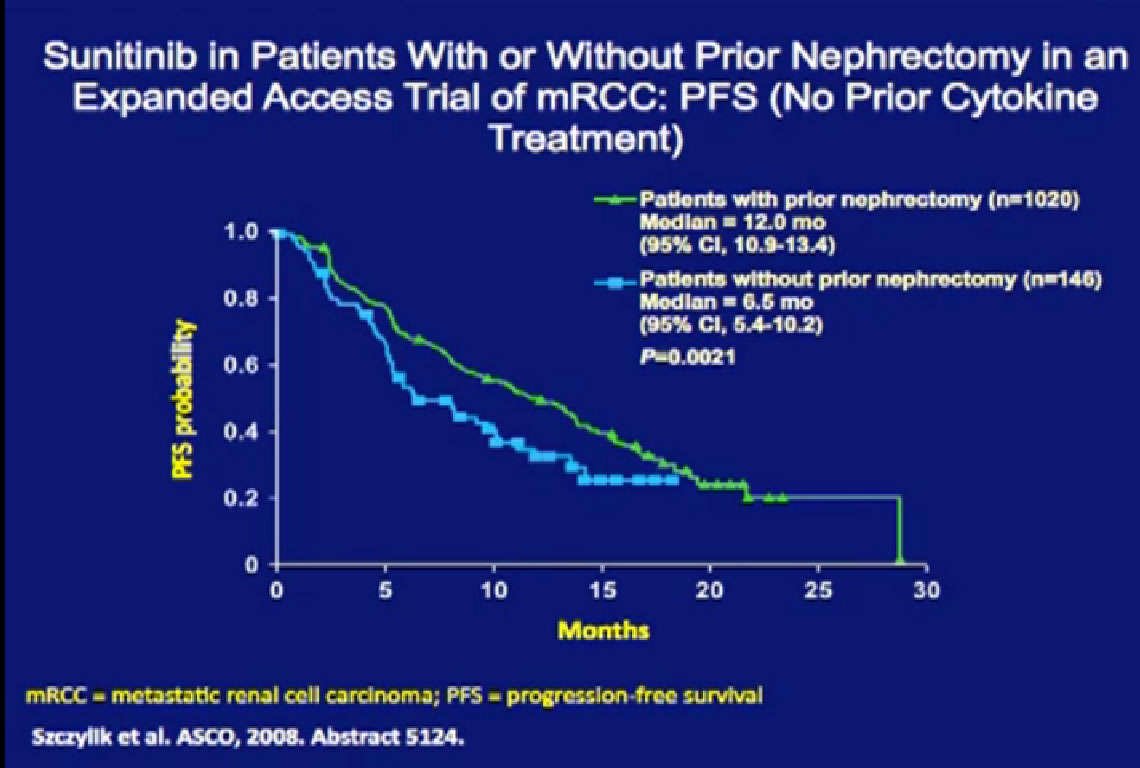
They also had a better progression free survival (PFS) and a better overall survival (OS) than patients treated with their primary tumors in place. Now this is retrospective, it is biased as it is not clear why the kidney was left in place for any one patient. That might have been because the patient was on death’s door and could not have had the kidney removed. But the data shows a benefit of being treated without having your kidney in place.
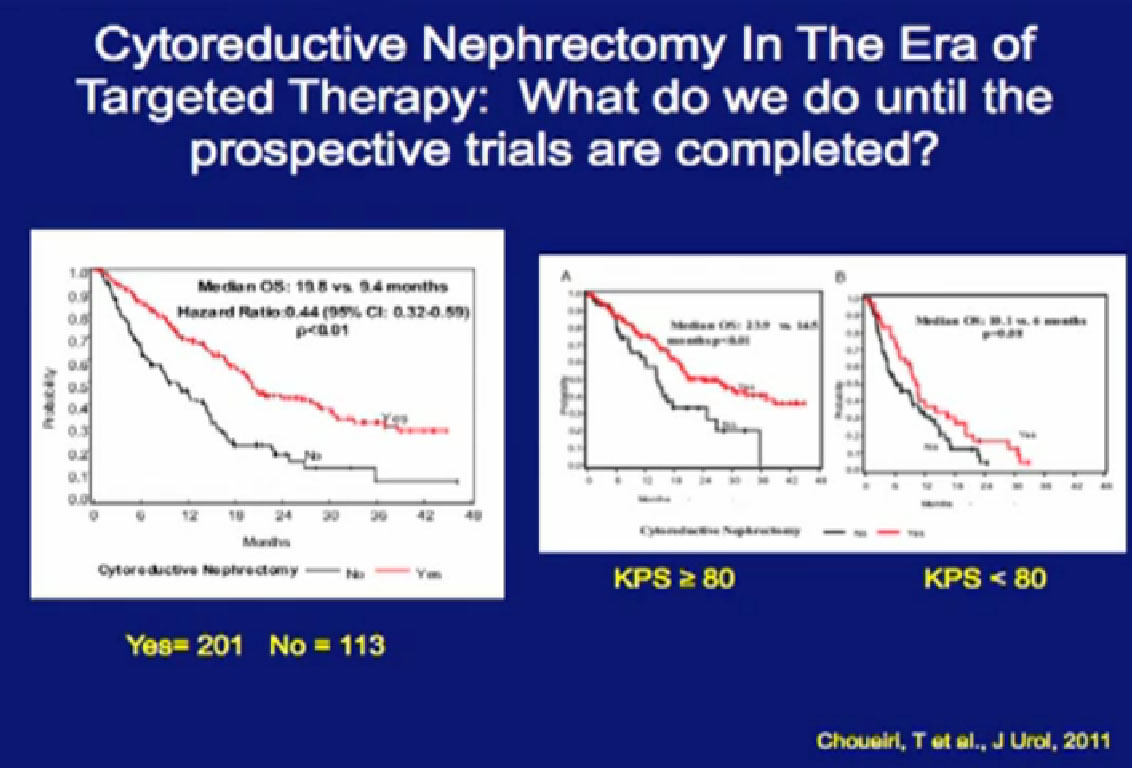
Newer data from Dr Toni Chouieri at Dana Farber compares are patients who presented with their primary tumors in place. Those who had the kidney removed prior to targeted therapy had a (overall) survival more than doubled compared to those treated with the primary tumor in place.
Those retrospective and (with patient selection) bias, it gives some guidance there may be some benefit to the removal of the kidney in the setting of targeted therapy.
Lecture continues as PART 2 with topic:
Cytoreductive Surgery for Metastatic RCC: It’s Not for Everyone