I was dying ten years ago. My kidney cancer had moved into my lungs, threatening to choke me to death.The tumor and kidney were gone, but 100s of tiny lung metastases were growing. Lucky to get an FDA-approved immune therapy, high dose interleukin 2, my own immune system was revved up so as to destroy the cancer. Thus, I am intrigued by all things about the immune system and cancer research. “Adaptive immunity in cancer immunology and therapeutics”is one of the most comprehensive explanation of the tumor cell/immune system interactions–that I can somewhat(!) understand.
http://ecancer.org/journal/8/full/441-adaptive-immunity-in-cancer-immunology-and-therapeutics.php
My summary is below, a more patient-friendly version. Don’t hesitate to take on the original, via the link! It is just the kind of article to take to your doctor to discuss immune response meds/treatments. It begins with the “abstract”, a summary of the information to follow.
Abstract: The vast genetic alterations characteristic of tumours produce a number of tumour antigens that enable the immune system to differentiate tumour cells from normal cells. Counter to this, tumour cells have developed mechanisms by which to evade host immunity in their constant quest for growth and survival. Tumour-associated antigens (TAAs) are one of the fundamental triggers of the immune response. They are important because they activate, via major histocompatibility complex (MHC), the T cell response, an important line of defense against tumourigenesis. However, the persistence of tumours despite host immunity implies that tumour cells develop immune avoidance. An example of this is the up-regulation of inhibitory immune monoclonal antibodies in clinical practice have been developed to target tumour-specific antigens. More recently there has been research in the down-regulation of immune checkpoint proteins as a way of increasing anti-tumour immunity.”
Immune Responses in Tumors—A Quick Summary by Peg
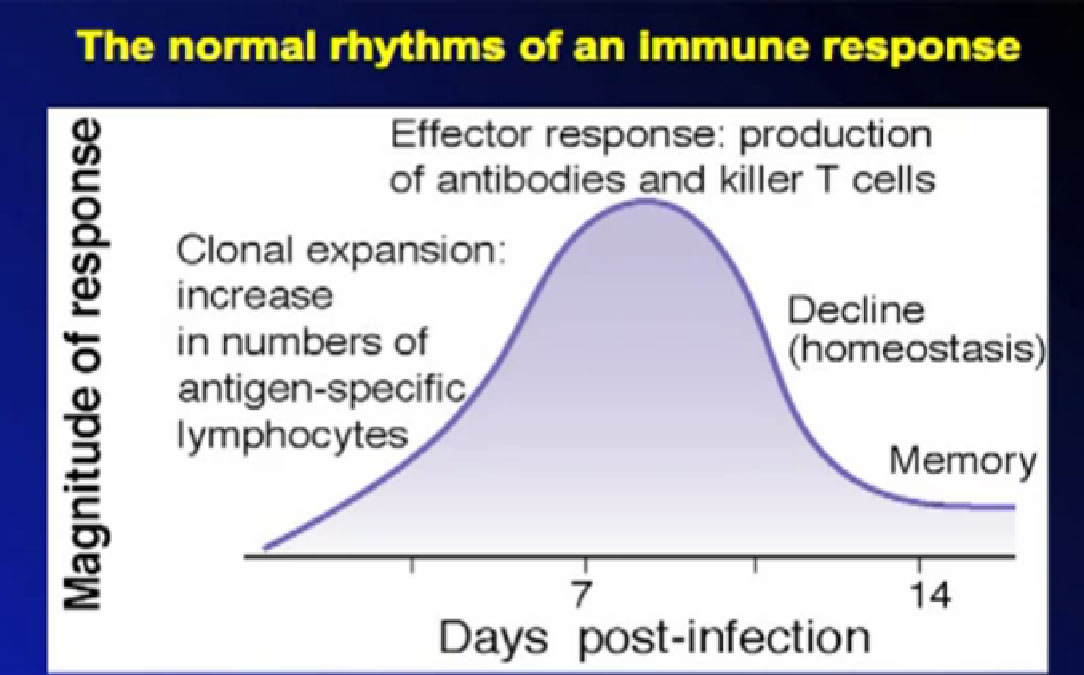
Since cancer cells are genetically different from normal cells, they also produce different substances—antigens—which can make them more noticeable to the immune system. Any antigen will generate a response from the immune system—think how the body reacts to an infection, an insect sting or a splinter.
Antigens trigger the immune system into action, keeping abnormal cells from taking over the system—most of the time. To grow, tumor cells develop inhibitory responses to limit or down-regulate those immune responses. An over-active immune response can be problem, well-known to those with severe allergies or auto-immune diseases like lupus. Keeping the proper balance is the norm for the immune system, despite ongoing external and internal changes
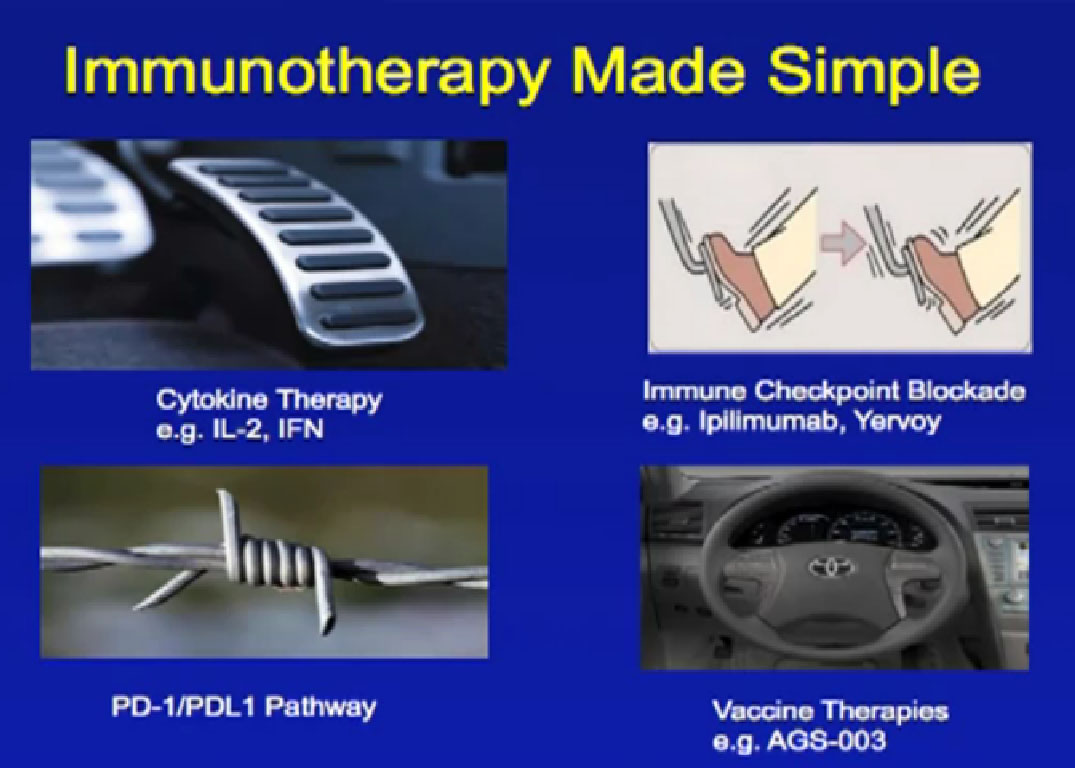
Using knowledge of these interactions to support the immune system, researchers have develop agent/medications. These are intended to strengthen the beneficial responses, and to prevent the tumors from suppressing or down-regulating those desired responses. Some monoclonal antibodies can effectively target these tumor-specific antigens and trigger tumor death or inhibit such growth. Some of these new agents include bevacizumab (Avastin), rituximab (Rituxin), alemtuzumab (Campath or Lemtrada), bortezomib (Velcade), denosumab (Xgeva) and trastuzumab (Herceptin), among many others, and for a variety of cancers.
Be aware that these agents may be called by the brand name, as Sutent, or the scientific name, as sunitinib, and may have several brand names for different cancers. Just another new challenge to all of us newbies.
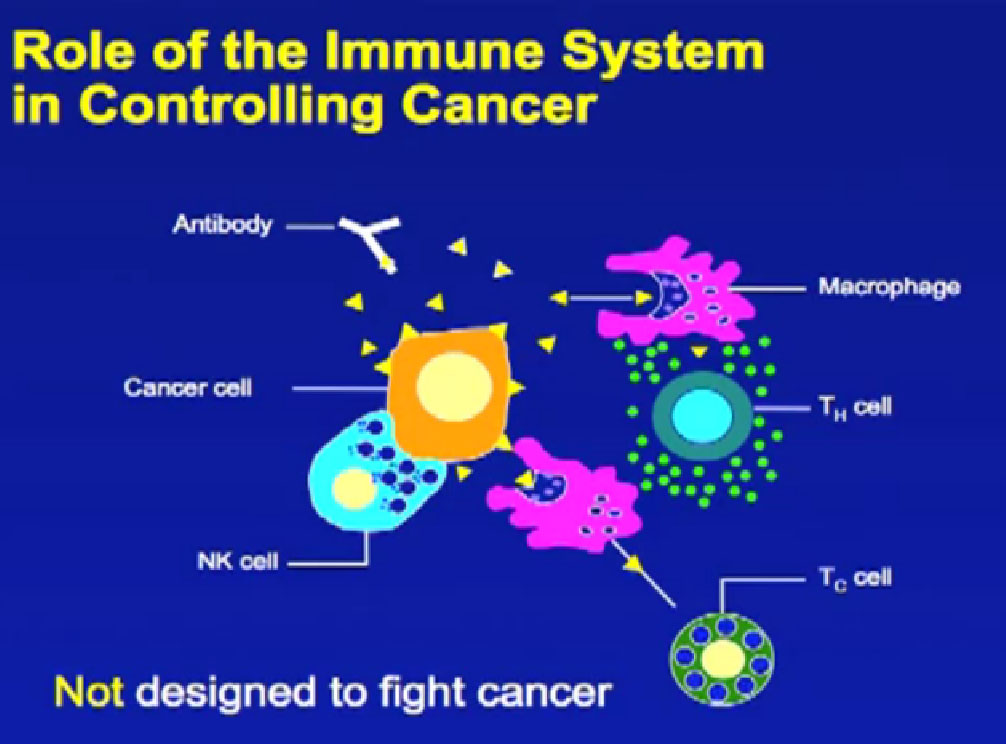
Tumors exist with a system of structures, various types of cells and with a chemical signaling process. These shifts away from the normal cells and organs produce tumor antigens. The immune system notices the antigens and works to destroy the foreign cells. Then the tumors shift to counter the immune response in an endless signaling battle. It is a dynamic “fail-safe” system, with multiple checks and balances, work-around pathways, evasive signaling, and constant testing to maintain itself. When this system does fail, a tumor can be established and move to different sites.
Solid tumors have a tumor core, a margin that is invading into a healthy structure–blood vessels or layers of an organ–and lymphoid components. This can vary patient to patient, despite the seeming similarity of tumors, and vary from one metastatic tumor site to another. Inside the tumor will be the immune-cell types–macrophages, dendritic cells, natural killer (NK) cells, mast cells, B cell, and T cells. Different immune cells can be found in different parts of the tumor, and the variation and the density of these cells may play a role in clinical response. It may be that this reflects the robust nature of the natural response to the tumor invasion, or reflect that the system is being overwhelmed by the tumor. Others think that the infiltration of immune cells can be utilized the support of the treatments given to the patient.
The linked journal article goes into detail as to the various types of responses, including adaptive immunity, immune editing and immune evasion. In summary, there are numerous approaches to limit tumor growth within the complex system of antigens and immune responses.
As immune cells infiltrate a tumor, that infiltration can be measured. What is the meaning of a higher or lower level of immune cell infiltration? The following paragraph sums up the challenge of using tumor infiltration as a marker of prognosis or treatment response.
It is a commonly held belief that infiltration of immune cells into tumor tissues and direct physical contact between tumor cells and infiltrated immune cells is associated with physical destruction of the tumor cells. That can reduce the tumor burden, and improve prognosis. An increasing number of studies, however, have suggested that aberrant infiltration of immune cells into tumor or normal tissues may promote tumor progression, invasion, and metastasis. Neither the primary reason for these contradictory observations, nor the mechanism for the reported diverse impact of tumor-infiltrating immune cells has been elucidated, making it difficult to judge the clinical implications of infiltration of immune cells within tumor tissues. J Cancer 2013; 4(1):84-95. doi:10.7150/jca.5482
Tumor Infiltrating Immune Cells—a Good Sign or Not?
If the immune system is at work, immune cells infiltrate the tumor to work directly against the tumor cells, is the tumor destroyed? Does the body naturally destroy the tumor? Does the patient benefit from medical treatments which support the immune system? Unfortunately, the presence of the tumor-infiltrating cells can mean very different things, with a better prognosis in one type of cancer, and a poorer prognosis in another.
Monoclonal antibodies can target antigens in blood cancers and solid tumors. In blood cancers, antibodies counter several cluster of differentiation (CD) markers, and in solid tumors, growth factors such as EGFR (epidermal growth factor receptor) or angiogenesis factors, such as vascular endothelial growth factor (VEGF). The mechanisms of action can lead to direct cell death, or simply impede its growth or inhibit checks on the immune response.
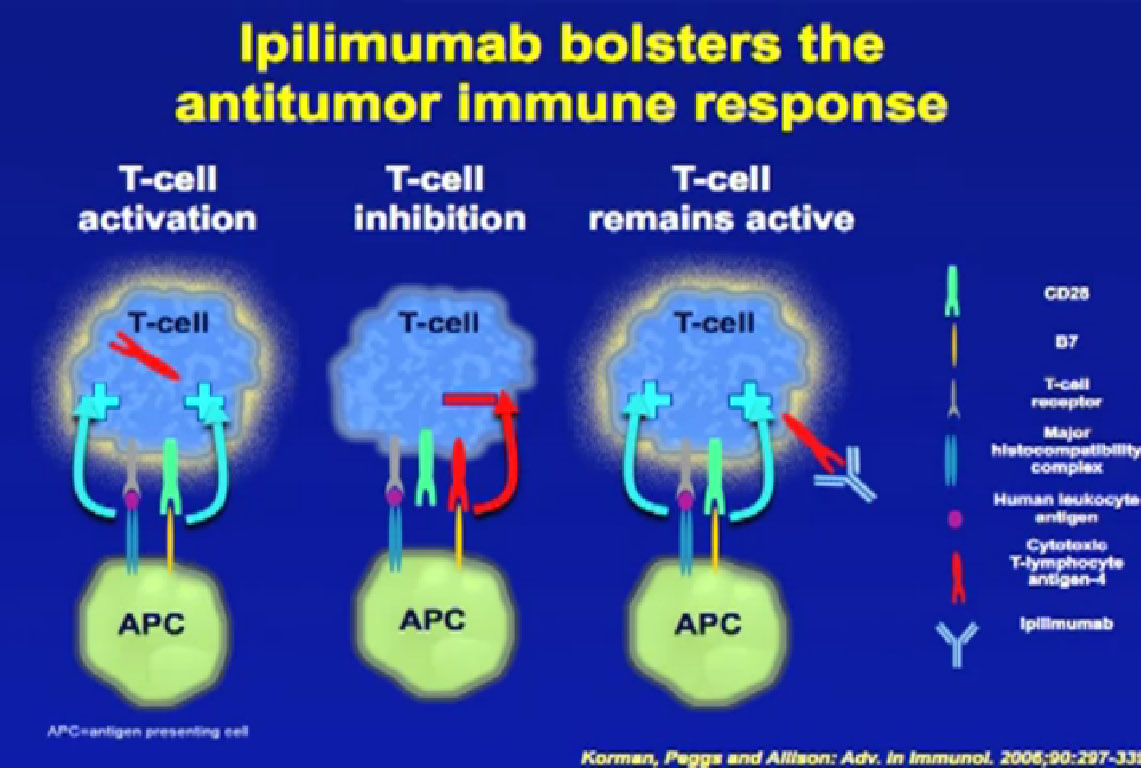
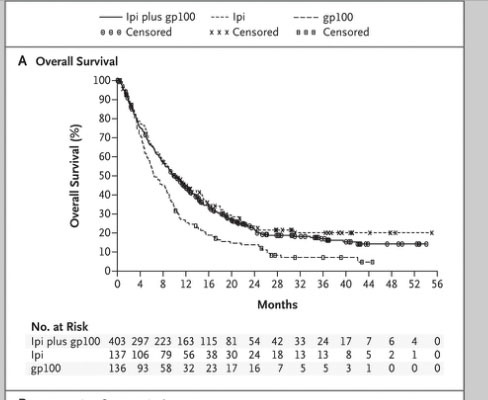
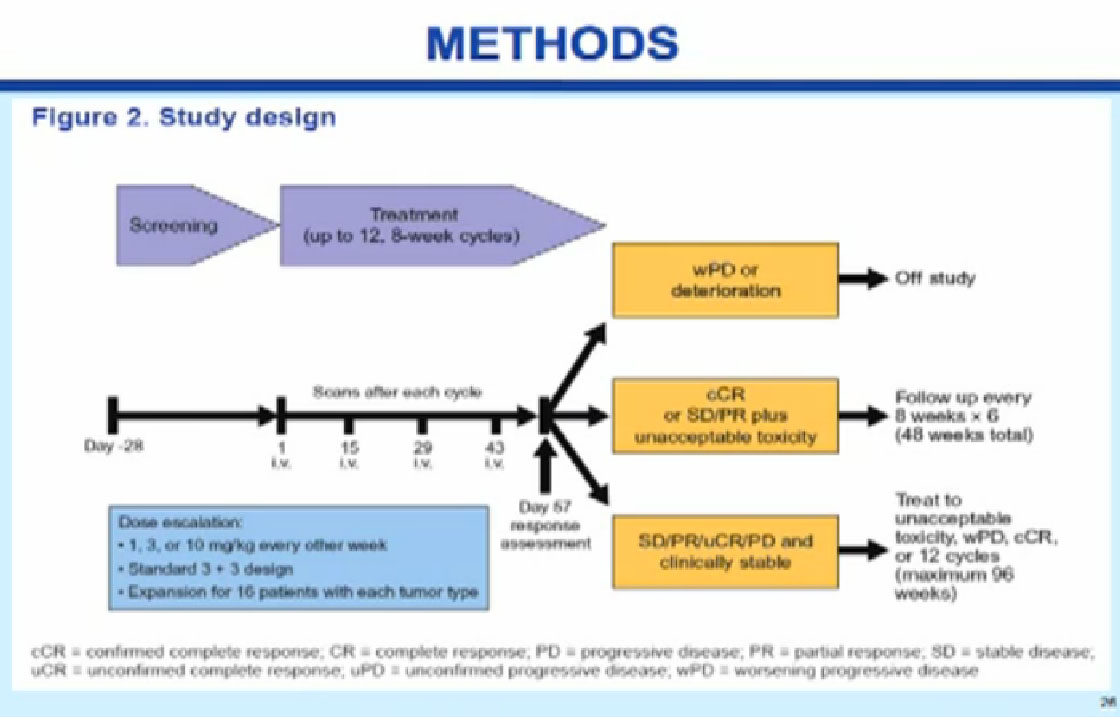
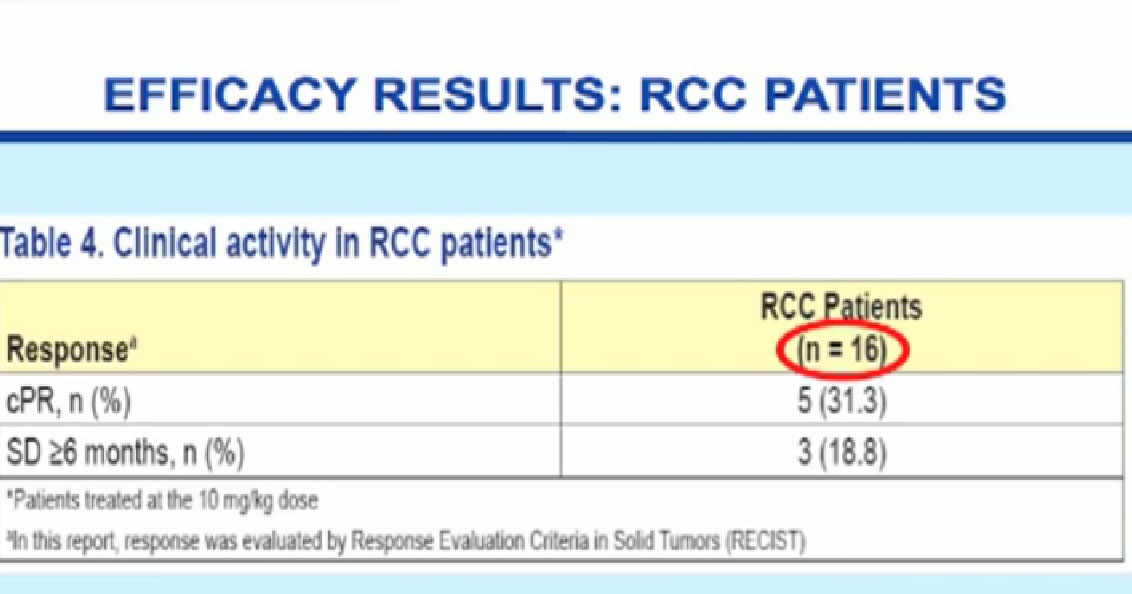
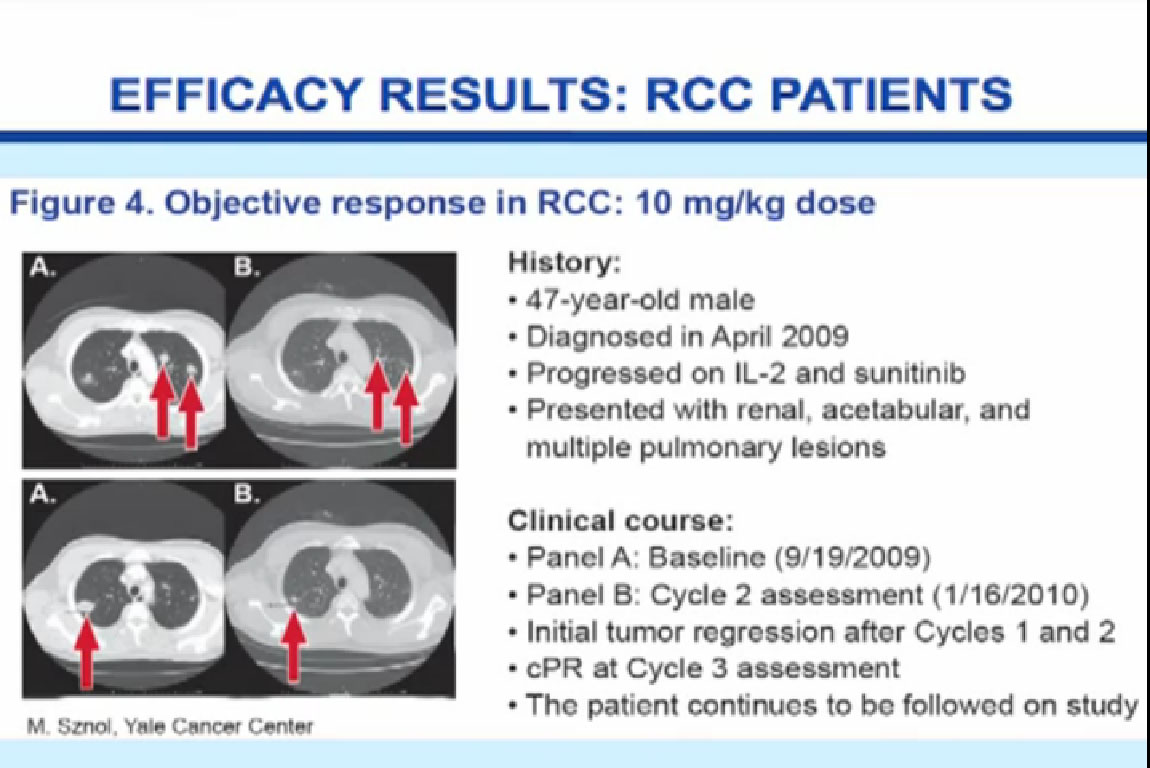
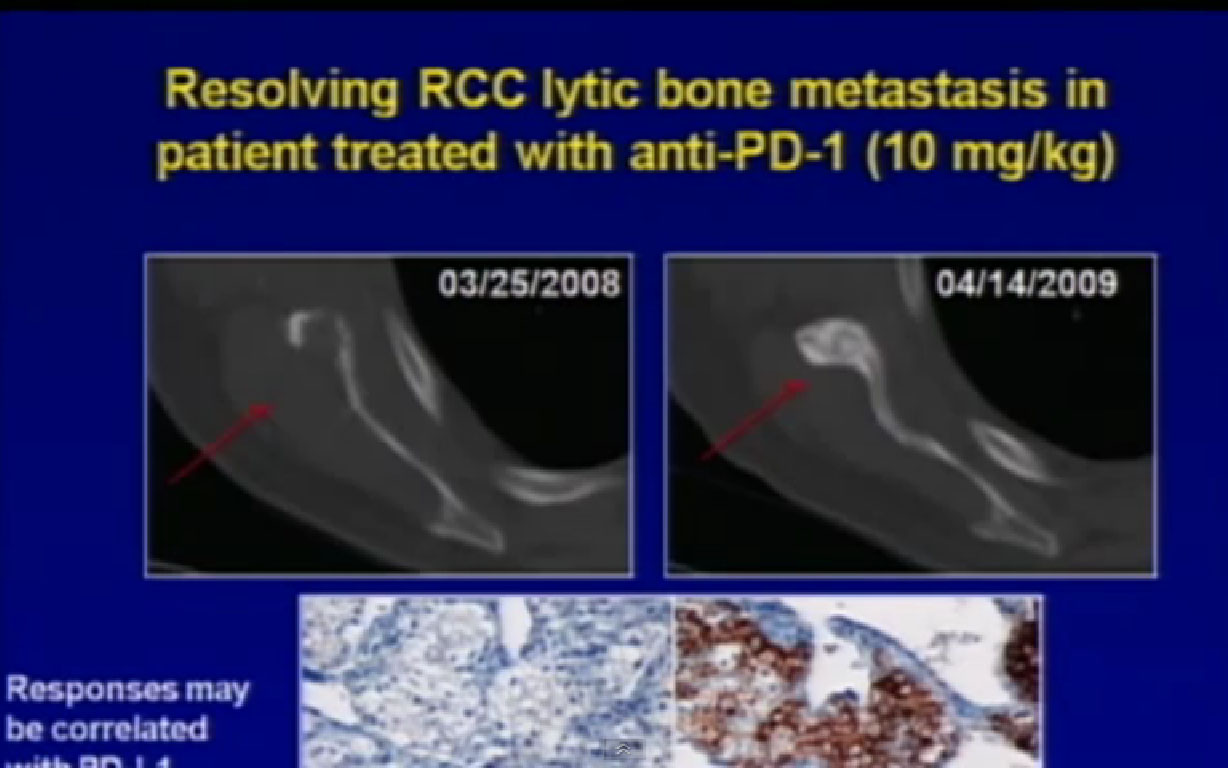
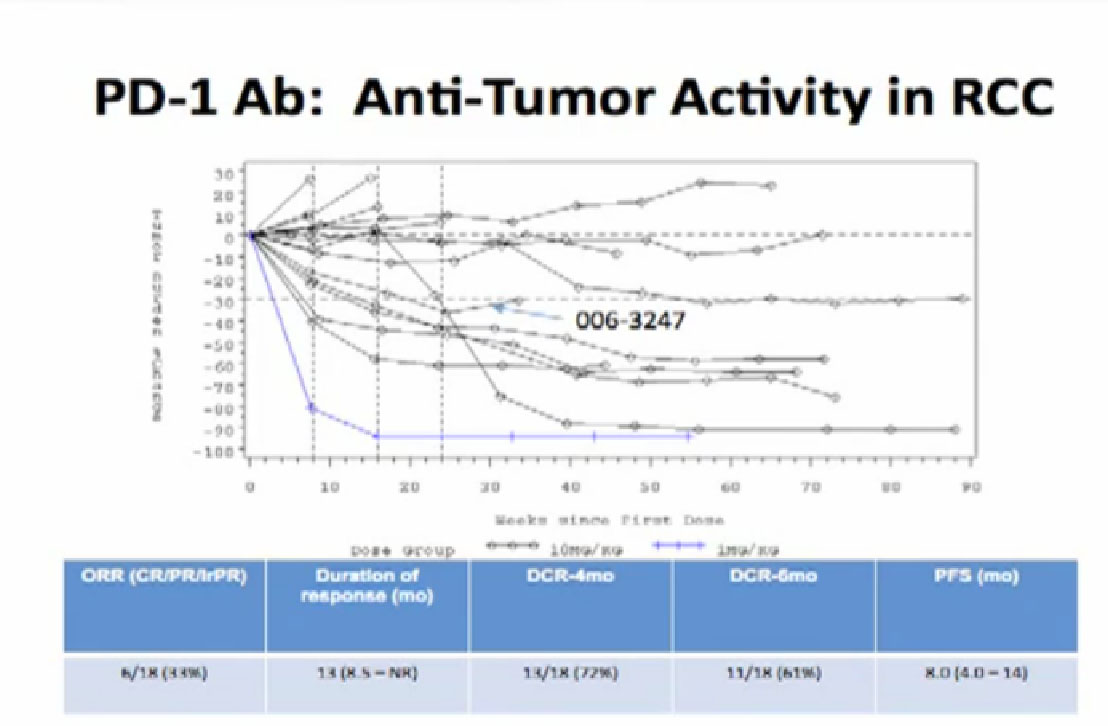
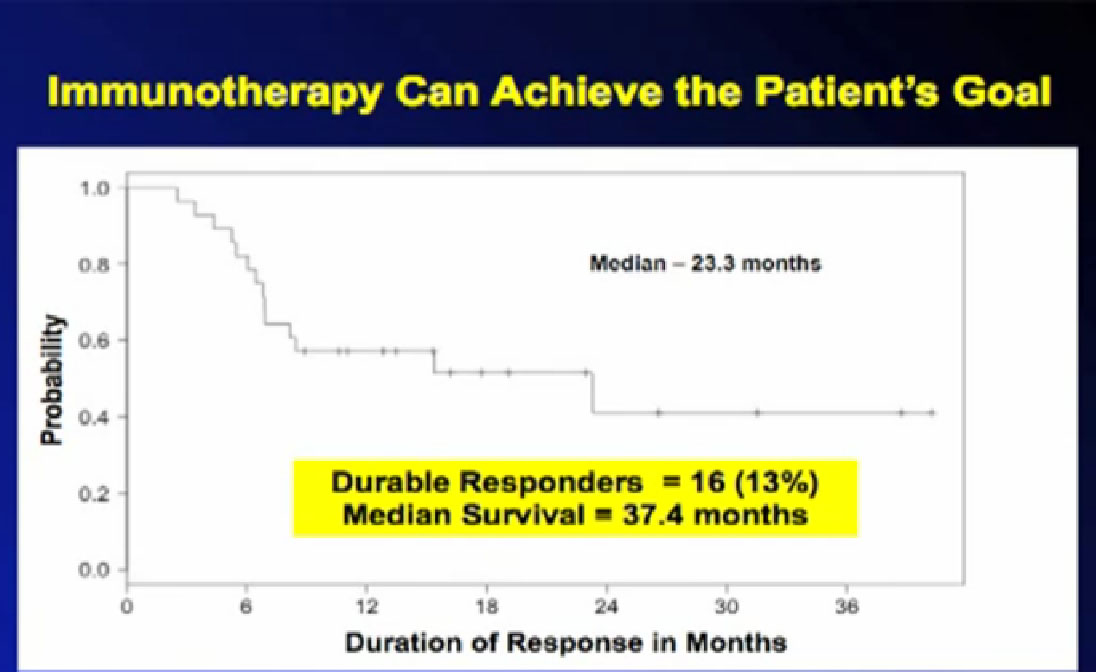
Normal cells are naturally programmed to die, but cancer cells do not “follow the program”. When certain proteins on the surface of cells bind with one another, the expected immune response is inhibited. These anti-PD-1 (anti-Programmed Death-1) proteins bind with other proteins, the binders or ligands, PD-L1 and PD-L2. Studies indicated these agents can help the immune system, with some disease stabilization or tumor shrinkage. Recent trials show some response by 20-25% of patients, some of whom had failed previous treatments. Some responses lasted more than a year. In a few cases, some responses were lasted for a period after stopping the medications. Newer trials will likely combine several of these therapies. This is not without risk, as some had severe side effects, and several patients died from such side effects.
Nevertheless, the earlier successes with this approach and the increased knowledge of the various immune responses to be targeted will continue, especially in combination studies. This work will have impact on existing immune therapies, as does the more integrated approach to cancer treatment.
I welcome any comments and corrections, and remind you that I am a patient, and am not a medical professional. My goal is to help educate other patients to receive the best understanding of their illness and best possible treatment.
Peggy Zuckerman
peggyzuckerman@gmail.com