Why should you care about genomic research? Simple; it could save your life! Want to know EXACTLY which type of cancer you have, and how to choose the best treatment? New hope comes from this research which really examines the nature of the cells that make up your cancer. Pretty important stuff.
Genomic research is bringing improvements to care, and points up the need to be aware of this new knowledge–and if your own doctor is keeping up with that type of data. At a 2012 Conference sponsored by the The Oncology Journal, Dr. Kimrym Rathmell spoke in regard the genomic knowledge that is leading to improved care for kidney cancer patients. Maybe the most critical lecture of late.
Dr. Rathmell begins after introductory remarks; Complete access on YouTube via this link:
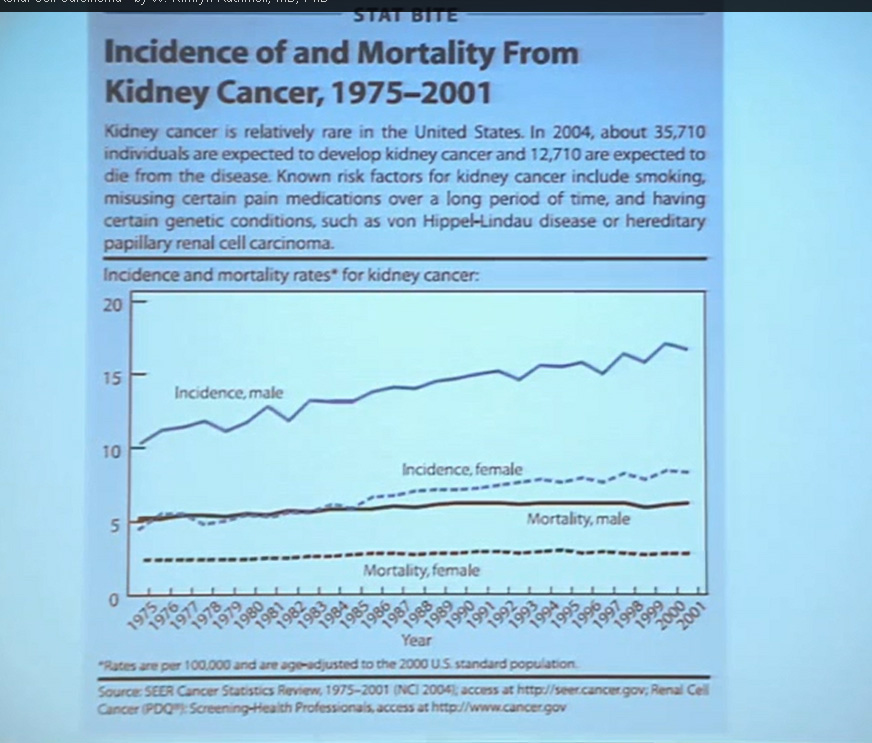
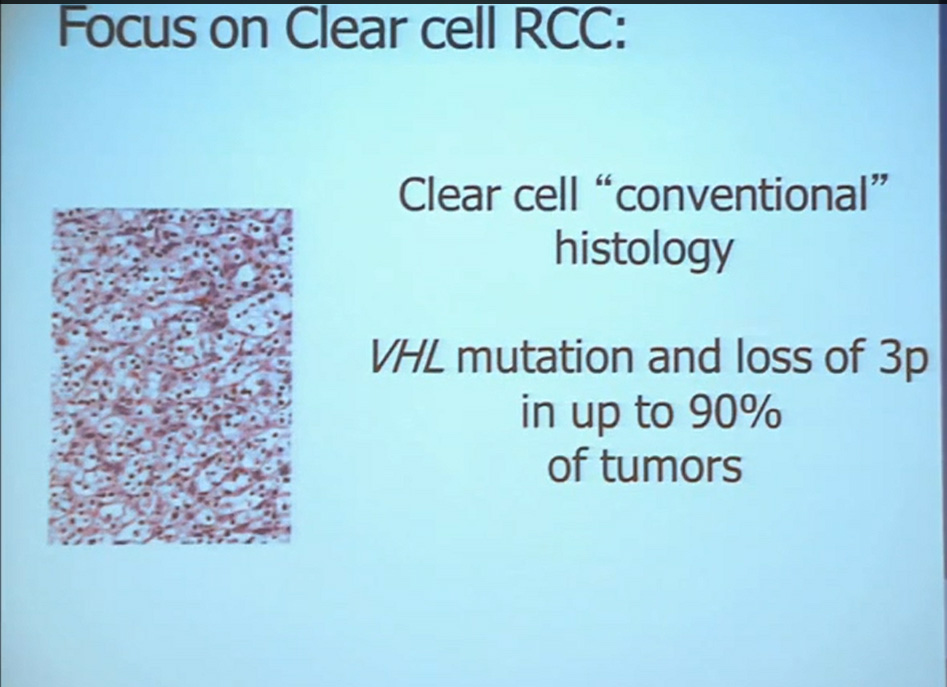
First, kidney cancer, like pancreatic cancer, has been on the rise. This is a somewhat dated slide, dating back to the 70s. We have seen a steady increase in this cancer. Although it was originally characterized as a rare tumor type, it is not really anymore.This talk will focus on one subtype of kidney cancer, that is, clear cell histology renal cell carcinoma. This is a histology slide showing why it is called clear cytoplasm
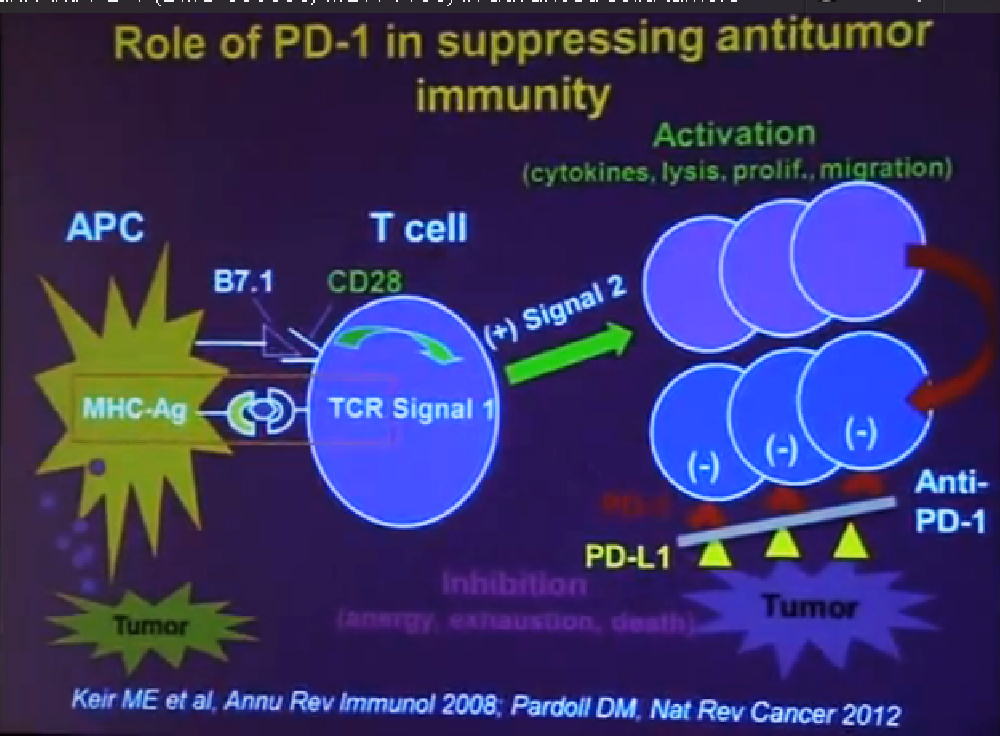
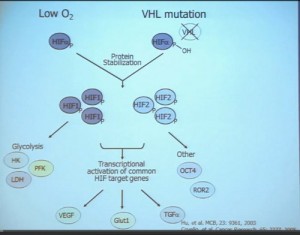
This tumor is characterized by particular mutation. That is the Von Hippel Landau gene, coordinate the loss of 3p, (a chromosome) where VHL is housed. We see these in mutations and loss of 3p which house other tumor suppressors as well, in up to 90% of these tumors. Based on this strong correlation between clear cell renal cell carcinoma and the VHL mutation, a tumor type. is a very distinct paradigm in which VHL loss causes upregulation of hypoxia inducible factors (HIF). These tumors are characterized by loss of high loss of these HIF factors. These are transcription factors that normally allow cells to respond to low levels of oxygen by turning on a repertoire of genes that allow them to bring in new blood vessels, to shift their metabolic properties, to migrate away, to promote survival, and to de-differentiate. That is a perfect storm for kidney cancer, in some respects.
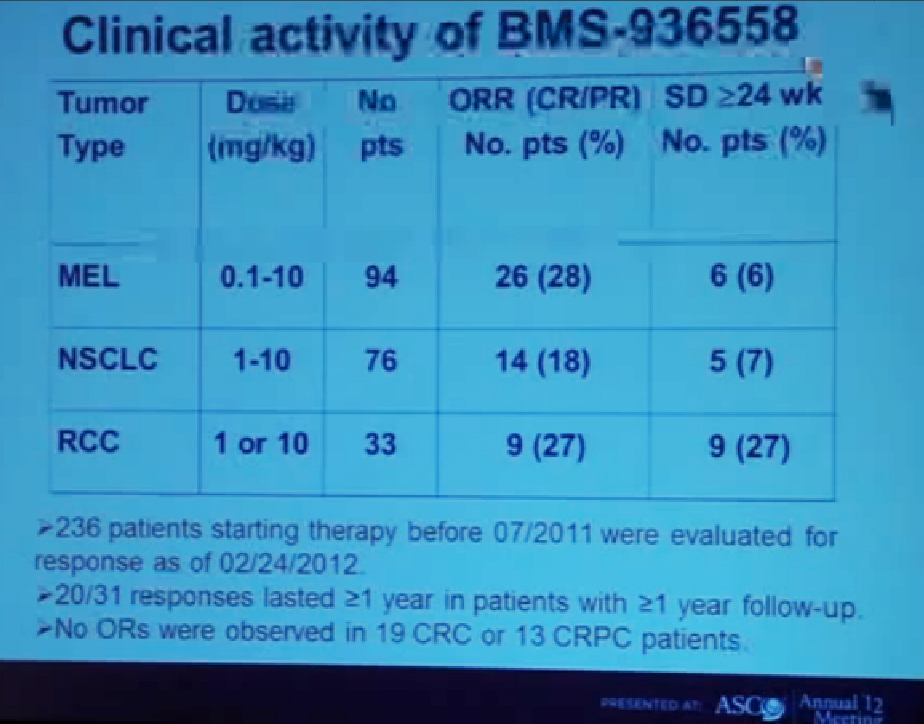
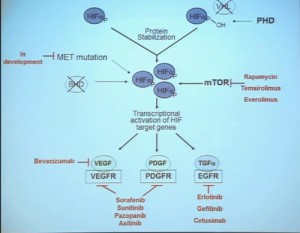
Because this cancer has highly nonresponsive to typical chemotherapy, there’s much effort in recent years to develop targeted agents. These targeted agents to date all focus on this well-known pathway in clear cell type renal cell carcinoma. Most of the agents focus far down on this pathway, including that of receptors of VEGF and PDGF. They are tyrosine kinase inhibitors, effective at reducing the tumor angiogenic profile and can be quite effective at reducing the bulk of these diseases. Other drugs similarly target this pathway, for example, targeting features of the tumor that enable HIF to be stabilized such as that in the mTOR pathways. Temsirolimus and Everolimus are approved for use. There are in-developments drugs for targeting MET, which is another mutation that can occur in this cancer, similarly increases HIF levels.
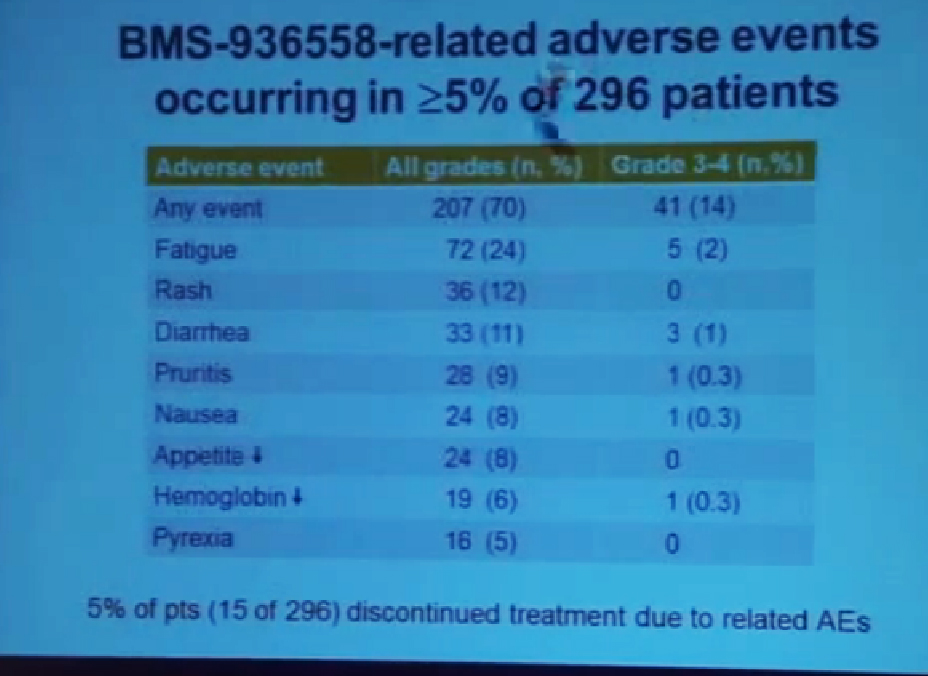
 But the reality of treating kidney cancer is that the available drugs that we have do not produce complete responses. We only work in the arena of minimal response and partial response. The extent of response that a patient gets is unpredictable. The duration is also unpredictable and the toxicity is also unpredictable. For drugs we expect them to be effective on average 1 to 2 years, this is chronic therapy, very expensive, and it’s dominated by effects that are substantially detrimental to quality-of-life—fatigue, rash, diarrhea, as well as laboratory abnormalities that indicate damage to the liver or elevations of glucose and cholesterol.
But the reality of treating kidney cancer is that the available drugs that we have do not produce complete responses. We only work in the arena of minimal response and partial response. The extent of response that a patient gets is unpredictable. The duration is also unpredictable and the toxicity is also unpredictable. For drugs we expect them to be effective on average 1 to 2 years, this is chronic therapy, very expensive, and it’s dominated by effects that are substantially detrimental to quality-of-life—fatigue, rash, diarrhea, as well as laboratory abnormalities that indicate damage to the liver or elevations of glucose and cholesterol.
PART I: Clear Cell Renal Cell Carcinoma, Molecular and Genetic Contributions to
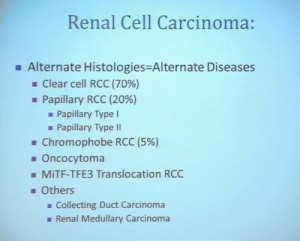
With that, I will talk about various molecular probes that we use to understand some of the diversity or the heterogeneity of these tumors across the clear cell renal cell carcinoma spectrum. Before I really dive into clear cell renal cell carcinoma, I need to point out that there are other histologies with this tumor as well. So when we say kidney cancer, we’re talking about a big spectrum. Clear cell renal cell carcinoma, we are talking about those tied to Von Hippel Lindau disease and loss of 3p and it is about 70% of all cases we encounter in cancers of the kidney. But there are also other types. Papillary type renal carcinoma, chromophobe, benign tumor—oncocytoma, a translocation form and some very rare. With these types of tumors we have very little in terms of knowledge of how to treat these patients. Their genetics are highly distinct from clear cell renal cell carcinoma. So someday in the future, we will understand not only how to treat not only our clear cell carcinoma patients, but how to use effective molecular information to target these cancers as well.
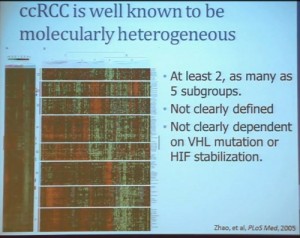
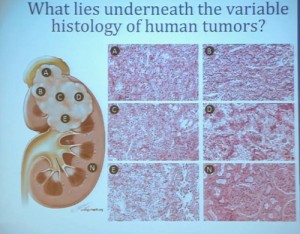
Clear cell carcinoma is well know to be molecularly heterogeneous for some time. This is a gene expression profile. We’ve already seen heat maps from several of these other talks, looking at gene expression profiles. And as you can see the gene expression profiles across a large selection of tumors here, suggests there are great areas of variability–at least two and as many as five groups, based upon gene expression purely.
Pattern Recognition Profile to Find Subtypes
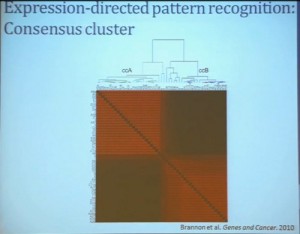
Our group undertook at the time developing a pattern recognition profile which is now fairly routine use. To try to see with a more robust computational strategy what subtypes we could really identify, that we could really pen down and understand with genetic profiles. 8aWe found two. For lack of better knowledge, we are calling clear cell A and clear cell B, ccA and ccB. These are very distinct biologically, and when we look at these tumors in terms of their outcome, they also have significant prognostic relevance–with the ccA tumors in this original cohort having a median survival of 103 months, compared to the 24 months for ccB tumors.
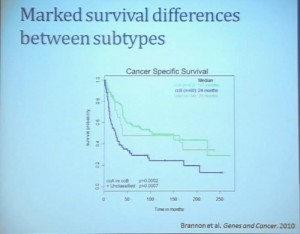 The TCGA which is been discussed here in many of the previous talks is a great source of validation. We assigned these tumors to clear cell A and cc B groups subtypes, validating our previous results with the clear cell A tumors having much better survival profile than those ccB tumors.
The TCGA which is been discussed here in many of the previous talks is a great source of validation. We assigned these tumors to clear cell A and cc B groups subtypes, validating our previous results with the clear cell A tumors having much better survival profile than those ccB tumors.
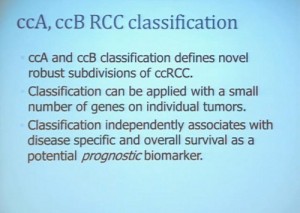
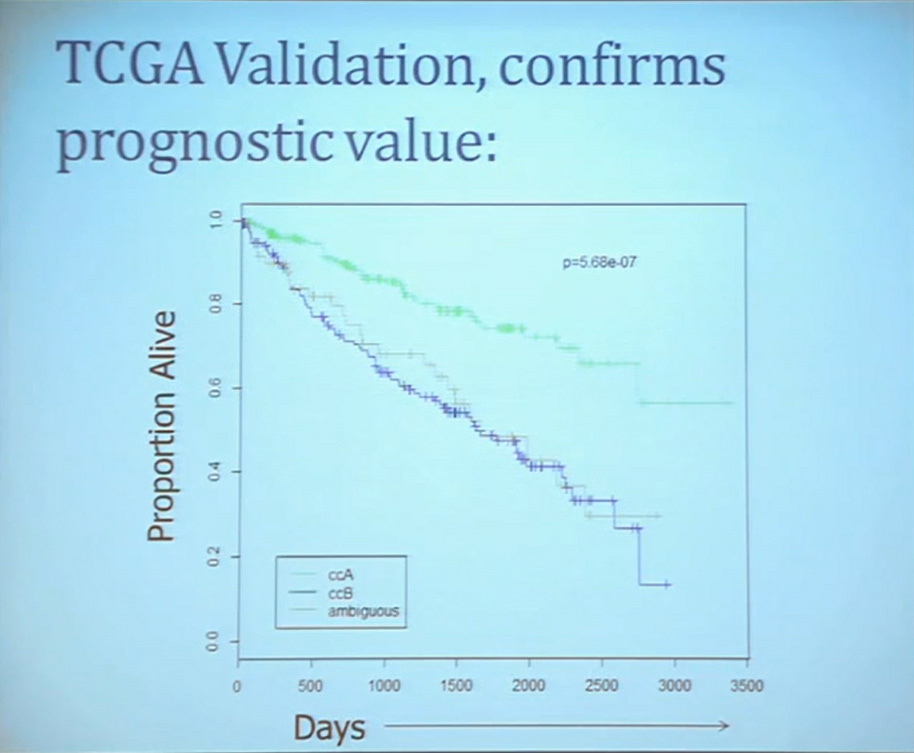 This classification scheme, which is based 120-130 gene signatures classified robust subdivisions of clear cell type renal cell can be applied with a small number of genes on individual tumors and is independently associated disease-specific overall survival, making it a valuable prognostic biomarker.
This classification scheme, which is based 120-130 gene signatures classified robust subdivisions of clear cell type renal cell can be applied with a small number of genes on individual tumors and is independently associated disease-specific overall survival, making it a valuable prognostic biomarker.
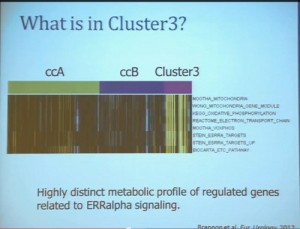
We use these profile tools to understand the rare variants. This is still in the clear cell renal cell carcinoma arena, but when we took a very large group of compiled tumors; this was a meta-analysis of 500 tumors, all histologically defined as clear cell type renal cell carcinoma, and we applied our expression pattern recognition algorithm. We asked for two groups and we found two and they correlated with our ccA and ccB, but when we ask for three groups, we can find a small group that now filters out. Now that we have power in numbers to identify what we called Cluster 3. What is in Cluster 3?
Cluster 3, as we’ve said, is histologically defined as clear cell renal cell carcinomas. But we look to the genetic expression profiles, it’s very different, particularly with regard to metabolic properties. We see upregulation of genes that are involved in mitochondrial regulation and oxidative phosphorylation, suggesting a striking difference in the way these tumors likely regulate metabolism.
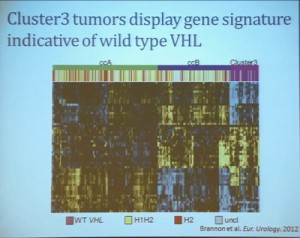
In addition, and now these are tumors that we can not go back and genotype for VHL mutation, for loss of chromosome 3p, but the loss VHL regulation leads to characteristic changes in the gene expression profile. So when we use the gene expression changes to predict whether these tumors have an intact VHL or a mutant type VHL. The wild type VHLS signature shown here is shown in purple. You can see that these purple tumors, the wild type VHL tumors all tightly cluster with Cluster 3. These are probably not clear cell renal cell carcinomas although many, pathologists call them that. So we pulled them all out so, asking, “DO they look a little bit different?” My graduate student, who did this work, came right away and said “There’s something funky about these clear cell tumors that we call Cluster 3.”
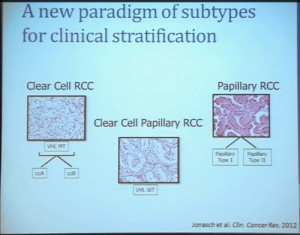
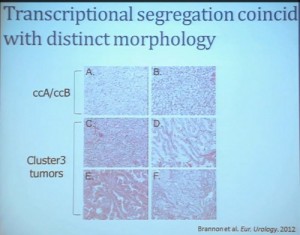
As you can see, these are clear cell A and a clear cell B tumors, but they all have the clear cytoplasm and really, what we are seeing, is that they are not distinct histologically, although they are very different molecularly. And as I have shown, they have a very different prognostic outcome. The Cluster 3 tumors; although the cells themselves might have clear cell cytoplasm that gave them the clear cell histology designation, they have a very different pattern of organization—with a papillary type of feature. So what we think it would be identified as is a new rare variant of clear cell renal cell carcinoma.
Simultaneously another group of pathologists identified, that the pathologists call clear cell papillary carcinoma. That suggests that we need to take a very great care as we treat these patients. What we have is clear cell type renal cell carcinoma, most of which are VHL-mutated, and we do have clear cell A and clear cell B. These are the tumors we should be treating with the drugs with identified, based on the effect of the pathway that is activated by loss of VHL. But clear cell papillary renal cell carcinomas probably won’t react very well, as they are VHL-wild type. Just like papillary renal cell carcinomas don’t react well either.
To summarize this section, clear cell renal carcinoma can be separated into ccA and ccB groups, based on transcript profiling, but further clustering can identify highly biologically dissimilar subtypes within the clear cell group, and that subtyping can convey a biological distinction which is a valuable tool for prognostic evaluation, and a likely cause of poor responses to some therapy.
Part III; Using Clinical Trials to Understand Biological Relationships to Response to Therapy
As my title indicated, we also refer to clinical trials to help us understand renal cell carcinoma a bit more. A clinical trial we completed some years ago, LCCC0603, was in neoadjuvant trial that looked at the treatment of renal tumors with sorafenib. Patients were identified as having renal tumor and underwent CT scans for basic size, description and PET scan, and then were treated with sorafenib. It is the first generation VEGF receptor tyrosine kinase inhibitor for 4 to 8 weeks, and then underwent post treatment CT scan, PET scan and a nephrectomy. We are going to look at radiographic indicators of response, rather than molecular indicators.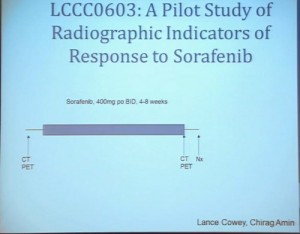
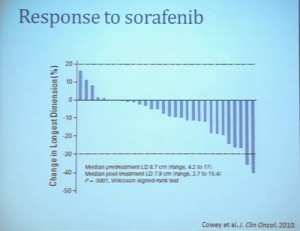
Waterfall Graph with Response at 30% as goal
First standard RECIST criteria do show that we do see partial responses. Again, there were no complete responses. Many had subpartial or minimal or some partial responses. Their tumors shrank, but most did not meet the standard criteria of 30% decrease in one longest size. Some tumors actually grew.
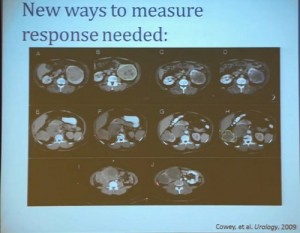
Now what we realized as we looked at these tumors, is that we probably need new ways to describe response. The standard RECIST criterion response is just based on longest diameter and measuring this in comparison, after treatment..
I will use this patient is an example. (References I and J, in the lowest row). Here is a pretreatment; we have a very large renal tumor. Post treatment, the tumor was still large, but it measures slightly larger than it had been before. But if you look at this tumor, it is very different. The central area of this tumor is now very dark, indicating necrosis—is what we think. But we took these tumors out, we could confirm that these dark areas were indeed necrotic. So we developed a new way to try to quantify the area of the tumor that is actually killed in response with this treatment.
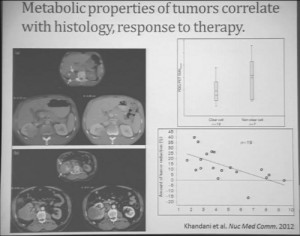
Similarly we were doing PET scans on these patients, and we were doing this because we’re trying to understand how the metabolic properties of these tumors might indicate how these tumors were likely to respond to this treatment. We see, and have known, that are some tumors which are very dim on FTG PET. This is a tumor; (Smallest of upper images) you can see that here is very visible on the PET scan. It doesn’t take up any FTG. So this tumor has the metabolic profile that is not dependent upon uptake of glucose. Others; this tumor (Smallest of lower picture), for example, have regional areas that are can be very high in terms of FTG uptake. When we looked at these tumors, we discovered first that non-clear histology tumors were much more likely to have high levels of FTG uptake. So, metabolically active tumors more likely in the (correction) non-clear cell group, probably the papillary, the chromophobes and the papillary clear cell types, than the clear cell group. Secondly. we discovered that the correlation between FTG uptake and response looked somewhat different than we might have expected. We might have expected the most metabolically active tumors would be those that would response better to anti-angiogenic agent. But the opposite was true. The best are those that had very low levels of uptake FTG uptake. We are still trying to understand exactly what that means. Certainly that means that those clear cell tumors are the ones more like to respond, activity, what we have known, but those are the ones with the lower level FTG activity. But we continue to try the metabolic properties of the tumors that make theme different more likely to respond.
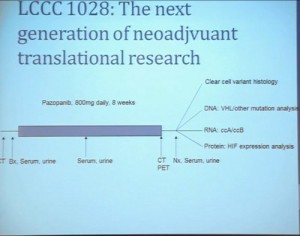 That leads to our next clinical trial. This is now ongoing. This is LCCC1028. It is a neoadjuvent clinical trial using the newest generation—well, they are coming out so fast that it’s the not the newest, but the next to newest VEGF receptor tyrosine kinase inhibitor. They are now getting PET scans and a biopsy to confirm in fact that they would be clear cell renal cell carcinoma, and also to allow us to do molecular studies that directly measure their metabolic activity and other effects. They’re being treated for eight weeks with another CT scan, undergoing nephrectomy. We will then be able to look at clear cell variant histology. They will all be clear cell going in, but there may be variants included–as well as looking at their VHL mutation and their other mutational status, their transcript profile, in particular the clear cell A and clear cell B group and other protein expression signatures.
That leads to our next clinical trial. This is now ongoing. This is LCCC1028. It is a neoadjuvent clinical trial using the newest generation—well, they are coming out so fast that it’s the not the newest, but the next to newest VEGF receptor tyrosine kinase inhibitor. They are now getting PET scans and a biopsy to confirm in fact that they would be clear cell renal cell carcinoma, and also to allow us to do molecular studies that directly measure their metabolic activity and other effects. They’re being treated for eight weeks with another CT scan, undergoing nephrectomy. We will then be able to look at clear cell variant histology. They will all be clear cell going in, but there may be variants included–as well as looking at their VHL mutation and their other mutational status, their transcript profile, in particular the clear cell A and clear cell B group and other protein expression signatures.
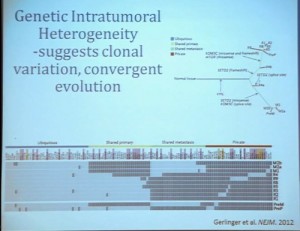
This, of course, is known for all tumors, but if you sample in multiple different places, the histology will look different and in effect, the grade can look different depending on where you are sample.
What does that mean molecularly? Well, a group at the Sanger Center published on a small number of tumors. When they sequenced these tumors, they found while there are some mutations that are ubiquitous, meaning the mutation is found in all samples across the primary tumor and the metastatic tumors, that there are mutations that are private. There are mutations that are common only among the primary tumors and there are mutations that are common only in the metastases, and there are a lot mutations that are unique to the individual sample. This makes a whole new level of complication as we moved toward personalized therapy, in particular therapy that is based on biopsy metrics.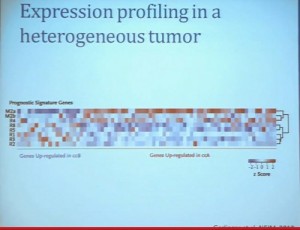
This group also looked at our clear cell A and B subtypes. And what they saw, when they looked at six samples from the primary tumors, was that in five of those samples, the gene signature indicated that these would be clear cell B type tumors. So depending on your glass half-full/glass half empty: The glass half full version of this, that five out of six times, they would pick that the patient would have poor outcomes. This patient has metastatic disease, so it fact, that is true. The glass half empty would be that one out of six times, he would pick wrong. This patient would have been indicated to have clear cell type A tumor, and you might have predicted that this patient would do well, when in fact that would be wrong. So what helps us understand the limitation of this test. It also gives us the opportunity to understand a little bit more about these tumors.
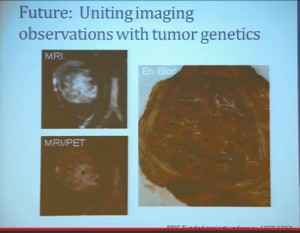
(Footnote reads: BRIC Funded Grants LCCC1213) So for the future, a trial (LCCC1213) that we have really just initiated is uniting some of these imaging observations we have made with genetics.
We are taking in patients. This is patient number one. Patient number two just has his MRI last week. and doing an MR in coordination with the PET scan so we can get the detailed look at these patients’ tissue perfusions, vascularity and the density of these tumors, as well as regional areas at the FTG uptake and sample according to the map that is created by the imaging, as well as samples that are collected, based on what we see grossly in the tumor. Here you can see a sample that we collected from a tumor region that is highly distinct from the mostly more pale yellow regions of tumor.
This is just begun, so I cannot tell how well were going to get to correlate the gene expression and genetic underpinnings, and what we see in the tumor and what we see in the MR / PET. But it will help us to move forward.
To summarize are multiple ways for RCC to diverge. The subsets can enrich tumor sets for clinical and genetic features, and a multiplatform approach that with genetics, molecular biology and imaging techniques will give us man ways to tackle a surprisingly very heterogeneous disease.



 QED
QED