Kidney Cancer Association National Patient Conference
Dr. Eric Jonasch; MD Anderson Cancer Center
April 14, 2012
Systemic Targeted Therapy for Metastatic Renal Cell Cancer in 2012
I am going to talk as systemic targeted therapy for Metastatic Renal Cell Cancer, what we are currently using and how we are using it, and the science about it, and how that leads us to new ideas, moving forward. Although I can see people here benefiting from what we do it is not enough.
When we talk about kidney cancer, we cannot talk about just one disease type. What I am going to talk about mainly is clear cell, and Dr. Tannir, my friend and colleague, is going to talk about the non-clear cell subtypes.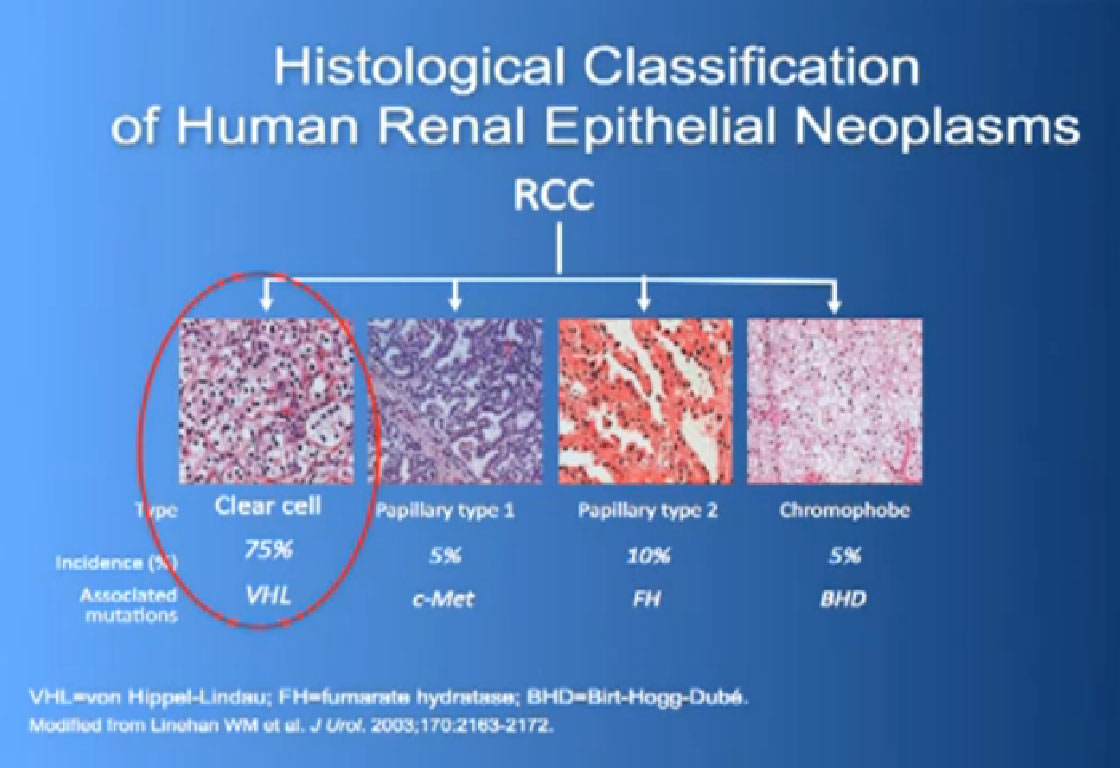
So what is ccRCC? It is essentially a cancer that looks like this under a microscope, and it was called this, back in the day. We more recently found that it has a mutation in the VHL gene (Von Hippel Lindau) in the large majority of individuals with and I also run a clinic where I try to marry the information we have about hereditary kidney cancer with non-hereditary kidney cancer to improved therapies.
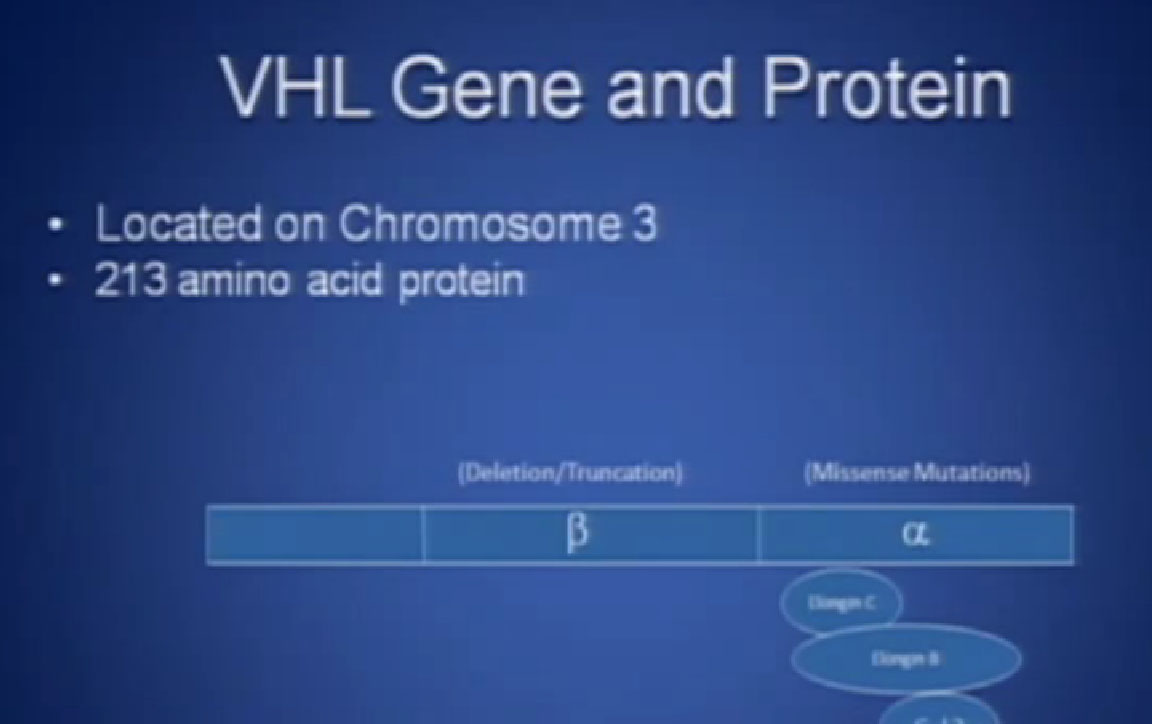 Slide 2: This is not to scale; it is 213 amino acids long and for the scientists in the audience, that is mercifully short, and it also has three exons in or three parts, or easier to study than most, but obviously still hard to study.
Slide 2: This is not to scale; it is 213 amino acids long and for the scientists in the audience, that is mercifully short, and it also has three exons in or three parts, or easier to study than most, but obviously still hard to study.
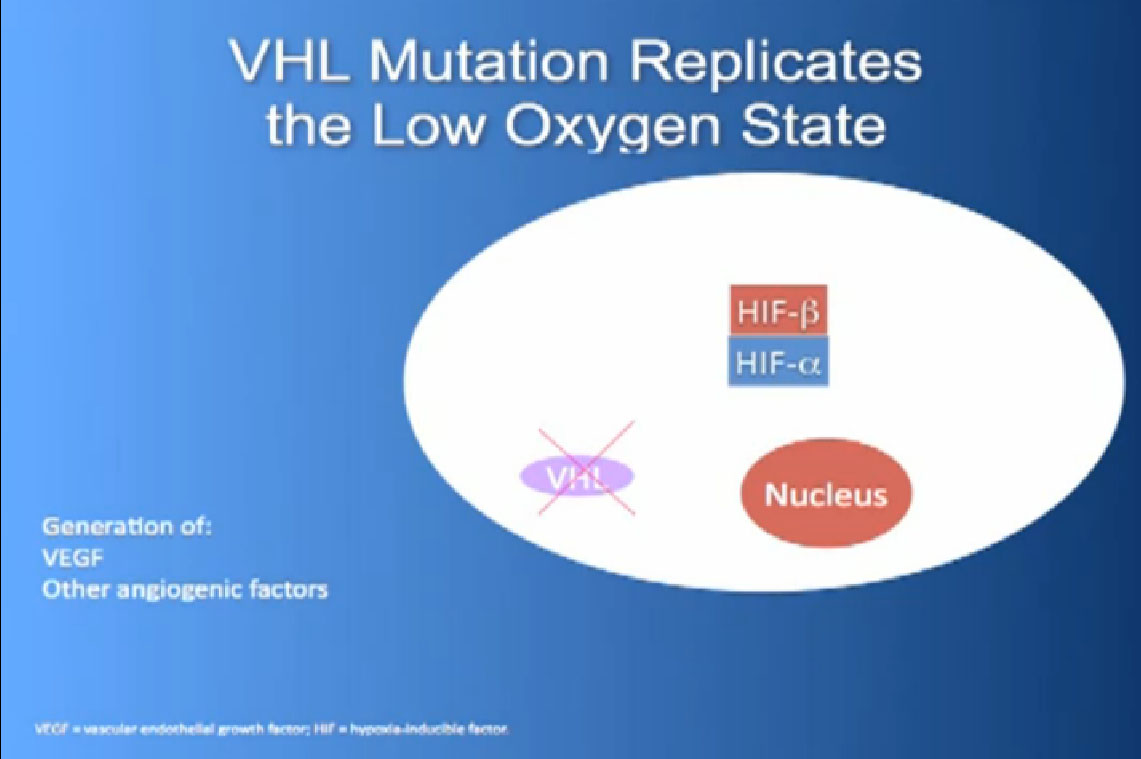
What it does, it essentially regulates how the cell reacts to oxygen. Obviously oxygen is our life’s blood, we need oxygen to, we need water, we need glucose. And our cells, if they feel like they hve enough need oxygen, they basically sit back and VHL will then take the transcription factor, it breaks it down If you have low oxygen state, then the cell will say, “Help, I need oxygen” and the VHL will step back, the transcription factors, HIF alpha and HIF beta are going to come together and you will get production of proteins, like VEGF which is a growth factor for blood vessels and some other things. If you have a mutation or something how an inactivation of the VHL gene, you have essentially this ongoing process of these cells, now cancerous, saying—somewhat untruthfully—we need more oxygen, we need more blood, build me an infrastructure.
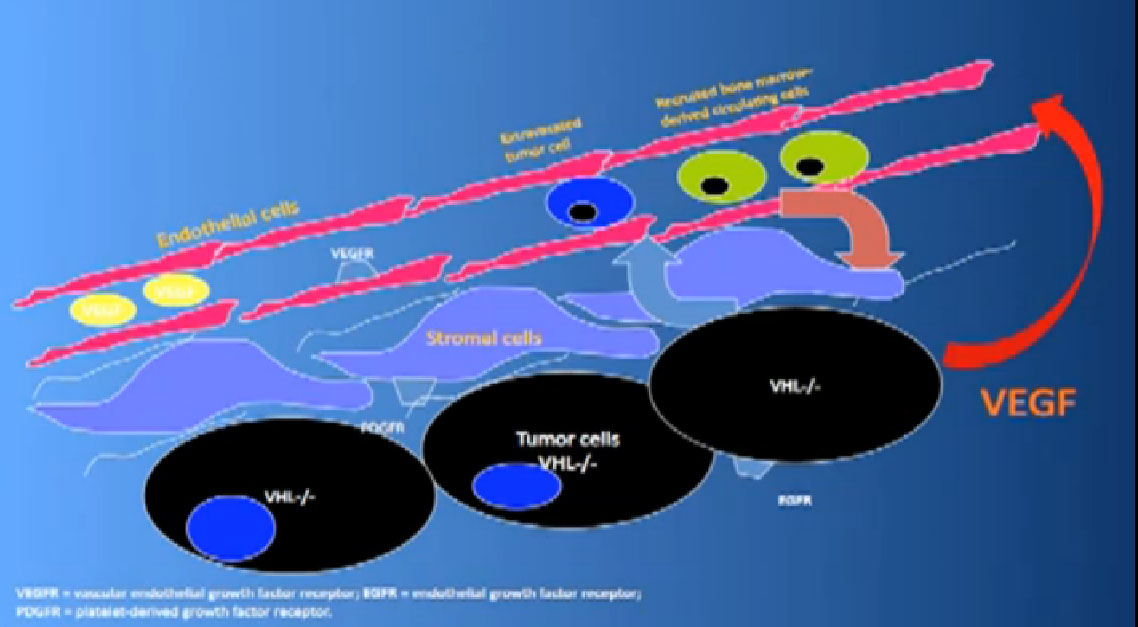
That infrastructure is as follows: When we think of cancer we can’t think of cancer cells The cancer cells are in black on slide and in blue above, the stromal cells–the “glue” cells, and above those the endothelial cells or blood vessel cells. This triumvirate plus other cells generate an organ—and it really is an organ—that we call cancer. And when we use therapies, we are blocking specific areas there.
I’ll tell you a bit about the therapies we use now and that some of you are on, and what they are actually blocking.
Here is some terminology you are about to hear, some jargon, as we talk about trials and therapies. 1) Progression free survival (PFS)—time it takes for cancer to start growing again and 2) Overall Survival (OS)—time it takes from start of treatment to passing of patient
 So the “blood vessel starving” are the five antiangiogentic therapies we currently have are listed here, and I am going to go through each of these in details.
So the “blood vessel starving” are the five antiangiogentic therapies we currently have are listed here, and I am going to go through each of these in details.
We also have some up and coming drugs. And the way these drugs work (Slide 4 again) is by blocking the blood vessels. They try to kill the blood vessels that feed the cancer. They don’t seem to have much effect on the cancer itself. That is why you have experienced that we can shrink this down, but we can’t make it go away. And what we need, and on our wish list, are kidney cancer cell-killing therapies for the future.
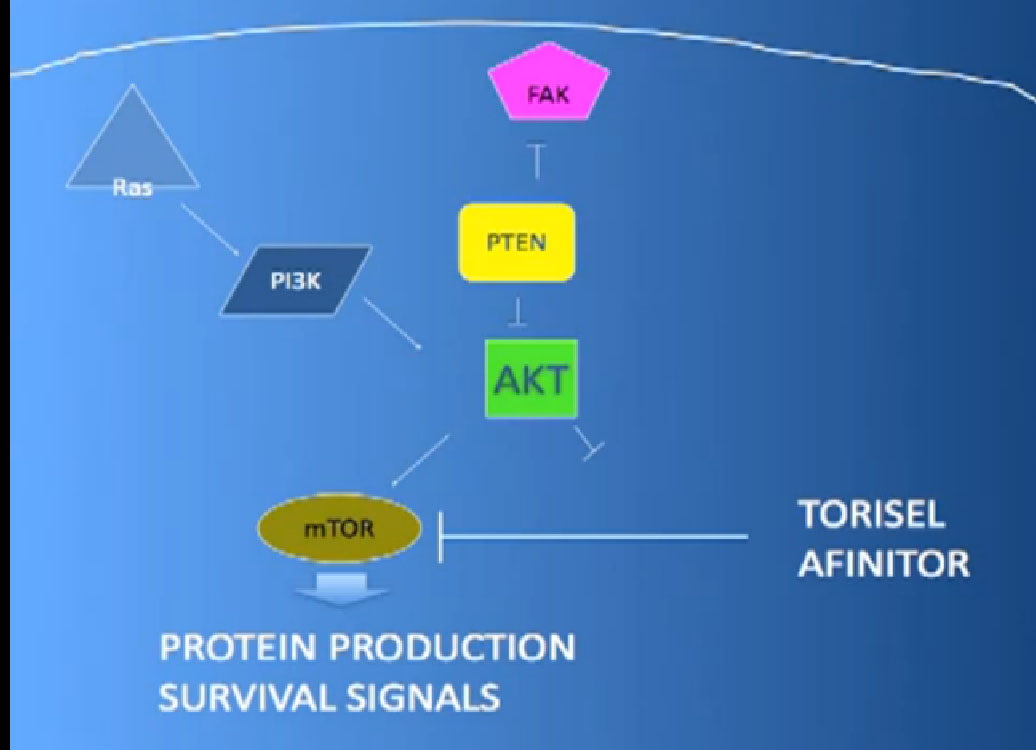
mTOR FDA approved;
Temsirolimus (Torisel)
Everolimus (Afinitor)
The mTOR inhibitors, of which there are two, Torisel and Afinitor, Temsirolimus and Everolimus.
What they do; they are actually working inside the cell both perhaps in the cancer and blood vessel cell. And they are blocking particular proteins that seem to be up regulated, overexcited, in the cancer cell that give them a selective growth advantage.
There we have on the bottom, kind of a brown color, mTOR, which if it is overactivated, results in production of and more survival … for the cancer cells—which is shouldn’t have. What Torisel and Afinitor do is to block that signal.

I am very interested in this article, but where is the rest of it? Thanks,
marci
Peggy, I would also like to read the rest of this…..did I miss it somewhere? Thanks Linda.